SHORT COMMUNICATION
Mosaicism for ATP2A2 Mutation and Mutant Allelic Fractions Detected by Droplet Digital PCR in Simple Segmental Darier Disease
Hiroshi KOGA1,2, Marie TAKAGI1, Kwesi TEYE2, Fumi KUWAHARA-SAKURADA1, Norito ISHII1,2, Takahiro HAMADA1* and Takekuni NAKAMA1,2
1Department of Dermatology, Kurume University School of Medicine, 67 Asahimachi, Kurume, Fukuoka 830-0011, Japan and 2Kurume University Institute of Cutaneous Cell Biology, Kurume, Japan. *E-mail: hamataka.1999@gmail.com
Citation: Acta Derm Venereol 2023; 103: adv12337. DOI https://doi.org/10.2340/actadv.v103.12337.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Jun 20, 2023; Published: Jul 13, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Darier disease (DD; Online Mendelian Inheritance in Man #124200) is an autosomal dominant disorder that presents in teenagers or adults, with multiple papules or plaques in seborrhoeic areas. Histology shows suprabasal acantholysis in the epidermis with overlying dyskeratotic cells. DD was caused by ATP2A2 mutations, which encodes the sacro/endoplasmic reticulum Ca2+ ATPase isoform 2 (SERCA2) (1). This protein serves to actively pump Ca2+ out of the cytoplasm and plays an important role in regulation of intracellular Ca2+ stores and subsequent epidermal cell-cell adhesion/differentiation.
The term mosaicism refers to the biological phenomenon in an individual with 2 or more genetically distinct cell populations derived from a single zygote. There are 2 patterns of mosaicism in autosomal dominant skin conditions, referred to as simple segmental mosaicism and superimposed mosaicism, previously termed type 1 and type 2 segmental mosaicism, respectively (2). Simple segmental mosaicism is caused by an autosomal dominant postzygotic mutation during embryogenesis in 1 allele of a gene that is otherwise normal in the affected individual, leading to mosaic skin involvements that correspond to the distribution of cells with the mutation. Superimposed mosaicism occurs in patients of generalized autosomal dominant genodermatosis with an additional postzygotic mutation that leads to loss of the normal allele, resulting in superimposed segmental manifestations. It may develop prior to the diffuse non-segmental disease. To date, the molecular basis of simple segmental DD has been reported in 6 cases (3–8). Superimposed mosaicism in DD is extremely rare, with only one published case in the literature (9).
We describe here the clinical and molecular characteristics in an additional Japanese woman with simple segmental DD, and the nature of underlying postzygotic mutation, which underscores its importance in clinical genetics.
CASE REPORT
A 57-year-old Japanese woman presented with a longer than 30-year history of keratotic papules and plaques that were limited to her left trunk and upper extremity (Fig. 1A). The patient was treated with topical steroid therapy without apparent benefits. Steroid therapy was discontinued, based on the patient’s personal reasons, and the lesions progressively increased in number following Blaschko’s lines. She had no history of neuropsychiatric abnormalities. No other family member, including her daughters, who were in their 30s, were affected. On initial presentation at the age of 57 years, a biopsy specimen was taken from her left anterior chest. Histology showed suprabasal acantholysis with dyskeratotic cells with eosinophilic cytoplasm (corps ronds) and focal hyperkeratosis with pyknotic retained nuclei (grains), confirming the diagnosis of segmental DD (Fig. 1B). Treatment with a combination of systemic retinoid (0.4 mg/kg/day) and topical maxacalcitol was started, and the skin lesions improved significantly. Over the course of 2 years, the patient has been maintained on a regimen of 0.2 mg/kg retinoid once a week to control the disease.
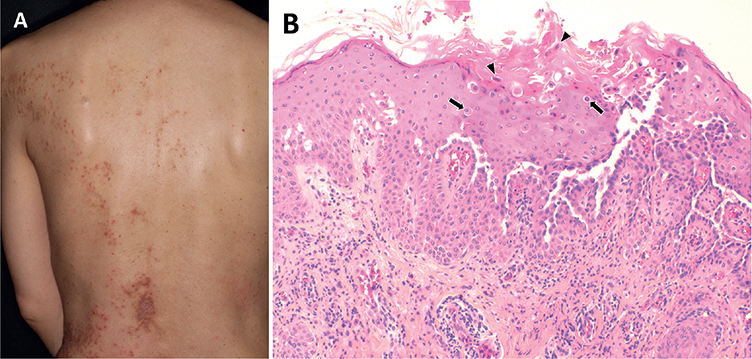
Fig. 1. Clinicopathological assessment of the current patient with simple segmental Darier disease. (A) Keratotic papules and plaques on the left side of her back following Blaschko’s lines. (B) Light microscopy reveals irregular acanthosis of the epidermis, suprabasal acantholytic dyskeratosis with corps ronds (arrows), and focal hyperkeratosis with grains (arrowheads; haematoxylin-eosin; original magnification ×200).
Materials and methods
After obtaining ethics approval and informed consent, genetic analysis was performed using genomic DNA from blood, the affected and unaffected skin of the patient in accordance with the principles of the Declaration of Helsinki. The blood sample was taken from the right median cubital vein of the unaffected side of the body. The affected skin of the left anterior chest was prepared originally for routine light microscopy and the unaffected skin was taken from the right anterior chest. Both skin tissues contained epidermis and dermis. The patient’s daughters were not available for any genetic investigations. For mutation analysis, all coding exons and situated in flanking introns of ATP2A2 were amplified by PCR using exon-specific primer pairs. Sanger sequencing was performed on an ABI 3130 DNA Sequencer (Applied Biosystems, Foster City, CA, USA). Droplet digital PCR (ddPCR) was performed on the Bio-Rad QX200 system (Bio-Rad Life Science, Hercules, CA, USA) using the ddPCR Supermix for Probes (No dUTP). Primers and probes were custom designed by Bio-Rad as ddPCR assay dHsaMDS457132439 based on the variant identified in Sanger sequencing. Two negative controls were included with each experiment. Data were analysed with QuantaSoft™ Analysis Pro (Bio-Rad).
RESULTS
Sanger sequencing revealed a heterozygous substitution in exon 1 of ATP2A2, c.68G>A, p.Gly23Glu (rs28929478) from the affected skin, whereas signals of the mutated allele were not visible in blood and the unaffected skin, suggesting that discrepancy of these results demonstrates the presence of postzygotic mutation in this patient (Fig. S1). The mutation has been previously reported in a case with generalized autosomal dominant DD (1).
The ddPCR was used to quantify the mutant allelic fractions (MAFs) in each sample of the patient. The MAFs in these tissues were 22.3% (affected skin), 0.22% (unaffected skin), and 0.52% (blood), respectively (Table I).
DISCUSSION
A few molecular approaches, including pyrosequencing and next-generation sequencing, have been utilized for quantifying MAFs (of 37% and 12.7–39%, respectively) in affected skins with simple segmental DD (4, 6–8). We confirmed the presence of ATP2A2 mutation via ddPCR in the affected skin from the current patient with MAF of 22.3%. This mosaicism level was relatively low compared with previous studies and sufficient to induce cutaneous DD lesions. It should be noted, however, that the MAF of the affected skin might be composed partially of mesenchymal cells originating from mesoderm (dermis and blood) relative to epidermis.
Sanger sequencing has limited sensitivity, as only mosaicism with MAF >10% can be identified effectively (10). Pyrosequencing and next-generation sequencing can detect a low-level mosaicism (MAFs in the range of 5% and 1–2%, respectively) (11, 12). Although ddPCR requires the development of a custom assay, validation, and optimization for a single known mutation and lacks the ability to screen the entire genome for novel mutations, it improves more accurately and sensitively to detect MAFs through counting mutation positive and negative DNA fragments in thousands of droplets using a single amplicon. Using ddPCR, we verified the presence of low-level postzygotic mosaicism in genomic DNA from blood and the unaffected skin in the current patient, at MAFs of 0.52% and 0.22%, respectively.
Assessment of somatic mosaicism across ectodermal (epidermis or oral epithelium), mesodermal (dermis, blood or saliva), and endodermal (urothelium) origin DNA can be useful to determine at what stage of embryogenesis the variant arose. In this study, the mutation is present in tissues derived from ectoderm (epidermis) and mesoderm (dermis and blood), although urothelial DNA from this patient was not available for molecular analysis. The mosaicism observed here can be explained by a mutational event before differentiation of the embryonic epiblast in early embryogenesis. Investigation of gonadal mosaicism in females is not as straightforward as in males, due to the invasive procedure required when collecting oocytes, part of extraembryonic mesoderm, which also arise from differentiation of the embryonic epiblast. These observations are important for the purpose of genetic counselling, because the present case implies a risk of simultaneous gonadal mosaicism that may cause the full-blown phenotype in the next generation. Since DD usually presents in teenagers or adults, there remains debate as to whether offspring for DD predisposition should be offered genetic testing for the disease risk. Genetic testing is a way to learn of risk status, prevent exacerbation of disease, and reduce anxiety, whereas perceived disadvantages include negative emotions associated with the test results.
REFERENCES
- Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet 1999; 21: 271–277.
- Happle R, Torrelo A. Superimposed mosaicism in tuberous sclerosis complex: a key to understanding all of the manifold manifestations? J Eur Acad Dermatol Venereol 2020; 34: 2511–2517.
- Wada T, Shirakata Y, Takahashi H, Murakami S, Iizuka H, Suzuki H, et al. A Japanese case of segmental Darier’s disease caused by mosaicism for the ATP2A2 mutation. Br J Dermatol 2003; 149: 185–188.
- Harboe TL, Willems P, Jespersgaard C, Mølgaard Poulsen ML, Sørensen FB, Bisgaard ML. Mosaicism in segmental Darier disease: an in-depth molecular analysis quantifying proportions of mutated alleles in various tissues. Dermatology 2011; 222: 292–296.
- Knopp EA, Saraceni C, Moss J, McNiff JM, Choate KA. Somatic ATP2A2 mutation in a case of papular acantholytic dyskeratosis: mosaic Darier disease. J Cutan Pathol 2015; 42: 853–857.
- Guerra L, Pedicelli C, Proto V, Condorelli AG, Mazzanti C, Castiglia D. A Postzygotic ATP2A2 novel mutation identified by next-generation sequencing in mosaic Darier sisease. Acta Derm Venereol 2019; 99: 115–116.
- Agematsu A, Kamata M, Uchida H, Nagata M, Fukaya S, Hayashi K, et al. A case of type 1 segmental Darier disease showing widespread Blaschkoid skin lesions with p.P160L mutation in ATP2A2. J Eur Acad Dermatol Venereol 2020; 34: e633–e635.
- Mohaghegh F, Youssefian L, Galehdari H, Tavakoli N, Vahidnezhad H, Uitto J. Whole-transcriptome sequencing identifies postzygotic ATP2A2 mutations in a patient misdiagnosed with herpes zoster, confirming the diagnosis of very late-onset segmental Darier disease. Exp Dermatol 2022; 31: 943–948.
- Fölster-Holst R, Nellen RG, Jensen JM, Poblete-Gutiérrez P, Steijlen PM, Schwarz T, et al. Molecular genetic support for the rule of dichotomy in type 2 segmental Darier disease. Br J Dermatol 2012; 166: 464–466.
- Cornec-Le Gall E, Audrézet MP, Le Meur Y, Chen JM, Férec C. Genetics and pathogenesis of autosomal dominant polycystic kidney disease: 20 years on. Hum Mutat 2014; 35: 1393–1406.
- Fu XJ, Nozu K, Kaito H, Ninchoji T, Morisada N, Nakanishi K, et al. Somatic mosaicism and variant frequency detected by next-generation sequencing in X-linked Alport syndrome. Eur J Hum Genet 2016; 24: 387–391.
- Fisher KE, Zhang L, Wang J, Smith GH, Newman S, Schneider TM, et al. Clinical validation and implementation of a targeted next-generation sequencing assay to detect somatic variants in non-small cell lung, melanoma, and gastrointestinal malignancies. J Mol Diagn 2016; 18: 299–315.