SHORT COMMUNICATION
First Use of Tapinarof Monotherapy for Seborrhoeic Dermatitis: A Case Report
Naiem T. ISSA1,2 and Michael KAISER3
1Forefront Dermatology, Vienna, VA, 2Issa Research and Consulting, LLC, Springfield, VA and 3Department of Dermatology & Cutaneous Surgery, University of Miami Miller School of Medicine Dr Phillip Frost, Miami, FL, USA. E-mail: Drnaiemissa@gmail.com
Citation: Acta Derm Venereol 2023; 103: adv12343. DOI: https://doi.org/10.2340/actadv.v103.12343.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: May 30, 2023; Published: Jun 27, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Seborrhoeic dermatitis is a common chronic and recurrent inflammatory skin condition with a significant negative impact on quality of life (1). It is characterized by erythematous, greasy-appearing, scaly yellow-orange plaques, typically involving the scalp, face, neck, chest or back (1). Symptoms include itching, pain, irritation and/or dryness which lead to physical and emotional discomfort. Global disease prevalence is estimated between 5% and 10% with approximately 3–10 out of 100 people being affected in the USA alone (2, 3). Current topical treatments include antifungal and anti-inflammatory agents, such as ketoconazole, clotrimazole, corticosteroids, and calcineurin inhibitors (2). We report here the first off-label use of the novel aryl hydrocarbon receptor (AHR) modulator, tapinarof cream 1%, for the treatment of seborrhoeic dermatitis of the face in a male patient.
CASE REPORT
A 39-year-old healthy male presented with long-standing history of seborrhoeic dermatitis of the face (Fig. 1A). The patient reported that the condition had been present for over 2 years. Previous treatments included several courses of topical antifungals (ketoconazole, clotrimazole) and corticosteroids (hydrocortisone, desonide, triamcinolone). These treatment courses provided intermittent relief, but he experienced frequent flares of scaling, erythema, and itch. He also expressed interest in topical non-steroidal treatment, due to concern about adverse effects and the wish to not undergo any systemic therapies.
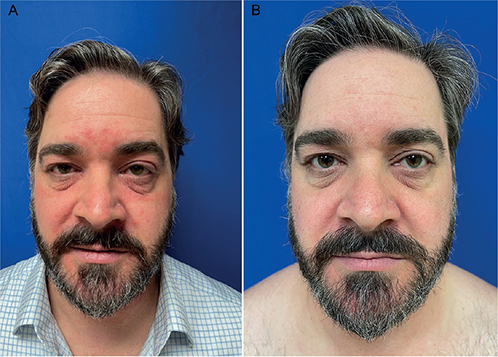
Fig. 1. Moderate-to-severe seborrhoeic dermatitis of the face. (A) At baseline. (B) One month after treatment with tapinarof cream 1% once daily. Permissions have been obtained to publish these photos.
The patient opted for off-label topical treatment with tapinarof, a novel anti-inflammatory treatment currently used for plaque psoriasis. He applied tapinarof cream 1% once daily to the affected areas of the face for 30 days. Clinical follow-up 1 month after treatment initiation showed significant clinical improvement and the patient reported no itching (Fig. 1B). The patient was then instructed to hold treatment for 1 month to assess for remittance. At the second follow-up visit 1 month later, the patient remained almost clear and reported no itching.
DISCUSSION
Off-label treatment with tapinarof cream 1% in this case was initiated based on its effectiveness in other inflammatory cutaneous conditions, such as psoriasis and atopic dermatitis (4). Tapinarof cream 1% was also utilized given its remittive effect in psoriasis patients (4).
The pathophysiology of seborrhoeic dermatitis hinges on 3 key factors: (i) secretion of lipids by sebaceous glands, (ii) colonization by fungal Malassezia species, and (iii) immunological predisposition of the patient (5). Malassezia fungi secrete lipases to hydrolyse the lipids into free fatty acids and lipid peroxides that cause a Th2 (T helper 2)- and Th17-driven inflammatory response with the production of numerous cytokines, such as interleukin (IL)-17 (5). These cytokines alter keratinocyte proliferation and differentiation, resulting in an impaired skin barrier and development of symptoms such as itching and pain.
Treatment of seborrhoeic dermatitis is aimed at reducing the proliferation of Malassezia fungal elements as well as the inflammation in response to them (6). As such, first-line therapies have been antifungal and anti-inflammatory agents. Topical antifungal medications include topical ketoconazole, ciclopirox, and miconazole. Topical anti-inflammatory agents include corticosteroids and calcineurin inhibitors. Keratolytic agents, such as tar and salicylic acid, are also used adjunctively (7).
Tapinarof is a novel, non-steroidal, first-in-class, topical small molecule aryl hydrocarbon receptor (AhR) agonist with anti-inflammatory effects. Tapinarof cream 1% was recently approved by the US Food and Drug Administration (FDA) for the treatment of adult plaque psoriasis and currently is being investigated in phase 3 trials for atopic dermatitis (8, 9). Furthermore, tapinarof downregulates IL-17 in lesional skin of psoriatic patients and exhibits a remittive effect where therapeutic response was durable for approximately 4 months of therapy in plaque psoriasis (10).
To date, tapinarof has not been investigated in the treatment of seborrhoeic dermatitis. Based on its ability to downregulate IL-17 and success in ameliorating other inflammatory skin conditions, we proposed that tapinarof may also be therapeutic in seborrhoeic dermatitis and exhibit a durable remittive effect. In the current case, tapinarof cream 1% was able to almost clear the patient’s moderate-to-severe seborrhoeic dermatitis, which affected his face, chest and back, and eradicate his itching. It also exhibited a 1-month remittive effect after stopping therapy, similar to its effect in patients with psoriasis.
We hypothesize that the therapeutic effect of tapinarof in this case is multifaceted. In addition to downregulating IL-17, tapinarof has been shown upregulate enzymes responsible for ceramide trafficking (such as UDP-glucose ceramide glucotransferase (UGCG)) and increasing the rate of keratinocyte differentiation, thereby altering skin barrier function (11). Other AhR ligands, such as indirubin, have also been shown to be effective for psoriasis, and it is possible that these ligands may also play a role in seborrhoeic dermatitis (12). Paradoxically, Malassezia spp. have been shown to produce potent metabolite activators of the AhR which may be implicated in the pathogenesis of seborrhoeic dermatitis (13). However, AhR agonists may exhibit distinct functions dependent on binding affinity, binding kinetics, location of binding within the AhR binding pocket, and cell-specific contextualization. It is currently unknown how exactly each AhR agonist may cause or ameliorate inflammation and remains a subject of intense study. Furthermore, incubation experiments of tapinarof with various microbe cultures suggest potential antimicrobial properties toward both fungi and gram-positive bacteria in an AhR-independent manner (14).
Given that seborrhoeic dermatitis is a common and frequently recurring inflammatory condition with negative impact on quality of life, we propose that use of the AhR agonist tapinarof results in rapid resolution and sustained remittive effect while avoiding the long-term side-effects of other topical treatments, such as cortico-steroids. More studies are warranted to investigate the efficacy and remittive effect of tapinarof in the treatment of seborrhoeic dermatitis.
In conclusion, seborrhoeic dermatitis is a prevalent condition typically treated with antifungal and anti-inflammatory agents. Quality of life may be significantly impacted in patients with frequently recurring disease despite numerous treatments. This case highlights the potential of the AhR agonist tapinarof to improve this condition, improve patient quality of life, and further decrease the need for frequent re-treatment given its remittive effect. While more studies and observations are needed to fully appreciate the potential of tapinarof for seborrhoeic dermatitis, this case highlights its potential as an emerging therapy.
REFERENCES
- Szepietowski JC, Reich A, Wesołowska–Szepietowska E, Baran E, Group NQoLiD. Quality of life in patients suffering from seborrheic dermatitis: influence of age, gender and education level. Mycoses 2009; 52: 357–363.
- Jackson JM, Alexis A, Zirwas M, Taylor S. Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol 2022 Dec 17 [Online ahead of print].
- Borda LJ, Wikramanayake TC. Seborrheic dermatitis and dandruff: a comprehensive review. J Clin Investig Dermatol 2015; 3: 10.13188/2373-1044.1000019.
- Keam SJ. Tapinarof cream 1%: first approval. Drugs 2022; 82: 1221–1228.
- Adalsteinsson JA, Kaushik S, Muzumdar S, Guttman–Yassky E, Ungar J. An update on the microbiology, immunology and genetics of seborrheic dermatitis. Exp Dermatol 2020; 29: 481–489.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol 2013; 6: 44.
- Kastarinen H, Okokon EO, Verbeek JH. Topical anti-inflammatory agents for seborrheic dermatitis of the face or scalp: summary of a Cochrane Review. JAMA Dermatol 2015; 151: 221–222.
- Nogueira S, Rodrigues MA, Vender R, Torres T. Tapinarof for the treatment of psoriasis. Dermatol Ther 2022; 35: e15931.
- Uppal SK, Chat VS, Kearns DG, Wu JJ. Topical agents currently in phase II or phase III trials for atopic dermatitis. J Drugs Dermatol 2020; 19: 956–959.
- Smith SH, Jayawickreme C, Rickard DJ, Nicodeme E, Bui T, Simmons C, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol 2017; 137: 2110–2119.
- Sutter CH, Azim S, Wang A, Bhuju J, Simpson AS, Uberoi A, et al. Ligand-activation of the aryl hydrocarbon receptor up-regulates epidermal UDP-glucose ceramide glucosyltransferase and glucosylceramides. J Invest Dermatol 2023 Mar 31 [online ahead of print].
- Sekhon S, Koo J. Indirubin: a novel topical agent in the treatment of psoriasis. Br J Dermatol 2018; 178: 21–21.
- Magiatis P, Pappas P, Gaitanis G, Mexia N, Melliou E, Galanou M, et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol 2013; 133: 2023–2030.
- Park HB, Goddard TN, Oh J, Patel J, Wei Z, Perez CE, et al. Bacterial autoimmune drug metabolism transforms an immunomodulator into structurally and functionally divergent antibiotics. Angewandte Chemie 2020; 132: 7945–7954.