ORIGINAL REPORT
Nocturnal Scratching and Quality of Sleep in Children with Atopic Dermatitis
Akiko SUGIYAMA1#, Yoko MURAKAMI2,3#, Kazuaki OKAMOTO4, Hiroshi NAKANO5, Masatoshi WAKATSUKI2, Toshiaki KAWANO2, Yuko AKAMINE2, Mihoko IWATA2, Chikako MOTOMURA2, Kaoru KOJIMA6, Makiko ODA6, Satoshi HONJO2,6, Hiroshi ODAJIMA2, Takeshi NAKAHARA7, Masutaka FURUE7 and Akihiko IKOMA4
1Department of Allergology and Dermatology and 2Department of Pediatrics, National Hospital Organization, Fukuoka National Hospital, Fukuoka, 3Department of Pediatrics, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital, Hiroshima, 4Maruho Co., Ltd. Osaka, 5Sleep Disorders Center and 6Department of Clinical Research, National Hospital Organization, Fukuoka National Hospital and 7Department of Dermatology, Kyushu University, Fukuoka, Japan
#These authors contributed equally.
Itching due to atopic dermatitis causes sleep disorders in children, but its pathology is unknown. The aim of this study is to investigate nocturnal scratching as an indirect index of itching during sleep and its relationship with depth of sleep in children with atopic dermatitis. Nocturnal scratching was measured in a total of 20 children with atopic dermatitis, using a smartwatch installed with the application Itch Tracker. Depth of sleep was analysed using polysomnography. The severity of atopic dermatitis was scored using Eczema Area and Severity Index (EASI) and Patient-Oriented Eczema Measure (POEM). The number and time of nocturnal scratching measured by Itch Tracker had a significantly positive correlation with EASI scores, whereas POEM scores were not correlated with EASI scores. Mean sleep efficiency was 90.0% and scratching episodes (n = 67) started mainly during the awake stage or light sleep stages. In the scratching episodes that started during sleep stages (n = 34), the sleep stage changed to a lighter one or to the awake stage in 35.5% of episodes. Itch Tracker is applicable to measure nocturnal scratching in children. Nocturnal scratching can deteriorate quality of sleep by changing the sleep stage to a lighter one or to the awake stage.
Key words: atopic dermatitis; itching; Itch Tracker; paediatric patients; polysomnography and scratching.
SIGNIFICANCE
This is the first study to simultaneously use Itch Tracker, a recently-developed app for measurement of nocturnal scratching, and polysomnography to assess nocturnal scratching and its influence on quality of sleep in children with atopic dermatitis. The results show a positive correlation between severity of atopic dermatitis and scratching, as well as indicating deterioration of quality of sleep during scratching in these children, adding to the evidence regarding quality of sleep impaired by itch in children with atopic dermatitis.
Citation: Acta Derm Venereol 2023; 103: 12345. DOI: https://doi.org/10.2340/actadv.v103.12345.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 22, 2023; Published: Oct 23, 2023
Corr: Yoko Murakami, Department of Pediatrics, Hiroshima Red Cross Hospital & Atomic-bomb Survivors Hospital, 1-9-6 Sendamachi, Naka-ku, Hiroshima City, 730-8619, Japan. E-mail: youkom12murakami@gmail.com
Competing interests and funding: Kazuaki Okamoto and Akihiko Ikoma are employed by Maruho Co., Ltd., the provider of the Itch Tracker app used in this study.
The other authors have no conflicts of interest to declare.
INTRODUCTION
Itching due to atopic dermatitis (AD) causes sleep disorders (1–6) and good quality sleep is one of the therapeutic goals in treatment of AD. Epidemiological studies indicate a positive association between AD and attention-deficit/hyperactivity disorder (ADHD) in children (7). It has been suggested that sleep disorders associated with itching due to AD may exacerbate the symptoms of ADHD in children (7–10).
In order to gain insights into sleep disorders in children with AD, it is crucial to clarify the pathology by which itching causes sleep disorders in children. However, it is difficult to accurately evaluate the severity of itching at night with commonly-used subjective assessments, such as visual analogue scale (VAS), by children or the observation of behaviour by their guardians. There is no objective method established to directly evaluate the severity of itching at night. Instead of a direct evaluation, the measurement of nocturnal scratching has been used by researchers as an indirect, but objective, index of itching severities at night, since itching induces the desire to scratch (11). Itch Tracker (Maruho Co., Ltd., Osaka, Japan) is a smartwatch application software with an algorithm to analyse acceleration data from Apple Watch (Apple Inc., Cupertino, CA, USA) that enables measurement of nocturnal scratching. This app has been shown to have high sensitivity and positive predictive values in the measurement of nocturnal scratching in adult patients with AD (12).
It is difficult to accurately evaluate the depth of sleep. The measurement of body movement using devices with accelerometers, such as actigraphy, is a broadly used method to differentiate sleeping and awaking status (13–18), but does not provide detailed information regarding depth of sleep. In addition, actigraphy does not seem to be optimal for evaluation of patients who scratch during sleep, since scratching might produce noise, which could be better used for analysis. On the other hand, polysomnography (PSG) with multiple parameters is a gold standard method to evaluate depth of sleep (19). PSG is more complex, but seems more appropriate than actigraphy for patients who scratch during sleep.
This study measured nocturnal scratching and depth of sleep in paediatric patients with different severities of AD, using Itch Tracker and PSG. The study focused on the relationship between the severity of AD and scratching as well as between depth of sleep and scratching.
MATERIALS AND METHODS
This study was approved by the ethics committee of Fukuoka National Hospital (approval number F30-21). All subjects at 6 years of age or older and their parents provided informed consent for participation in writing. Parental consent only was obtained for children under 6 years of age. The study was in 3 parts, as described below.
Part 1
The relationship between the severity of AD and the time and number of nocturnal scratching was examined in 14 paediatric patients with AD (mean ± standard deviation 9.2 ± 4.3 years old, 9 males and 5 females). The severity of AD was always assessed by the same 2 physicians together (1 dermatologist and 1 paediatrician who both have expertise in paediatric dermatology) using the Eczema Area and Severity Index (EASI). The number of scratching episodes from bedtime to waking up and the duration of each scratching episode were measured for 1 night at home by wearing an Apple Watch installed with the Itch Tracker app during the night. The Itch Tracker app was used with the knowledge that it has not been validated for children, which implies a potential risk of lower accuracy in measurement than for adults. In addition, patients were asked to evaluate subjective symptoms using Patient-Oriented Eczema Measure (POEM) (20–23). If patients were unable to self-evaluate, their parents were asked to provide evaluation.
Part 2
Of the patients participating in Part 1, the study followed up 6 children who agreed to further measurement and revisits for a maximum of 3 visits regarding the severity of AD, nocturnal scratching and subjective symptoms. The interval of follow-up visits was 6–31 days. The subjects were provided with standard medical treatment and guidance regarding daily life depending on the severities and symptoms.
Part 3
Six children underwent a standard overnight PSG in the hospital. An experienced physician of the paediatric Sleep Disorders Center at National Hospital Organization, Fukuoka National Hospital, Fukuoka, Japan analysed the PSG data and determined the sleep stage. PSG data were recorded using an original polygraph system based on an electroencephalography system (EEG-1200; Nihon Kohden, Kobe, Japan), in which electroencephalography (C3-A2, O2-A1), bilateral electrooculography, submental electromyography, electrocardiography, and bilateral anterior tibial electromyography were performed. Oronasal airflow was monitored using a thermocouple sensor and a nasal prong pressure transducer (PTAF; Pro-Tec, Mukilteo, WA, USA). Thoracic and abdominal respiratory movements were monitored using respiratory inductive plethysmography (RIP; Inductotrace; Ambulatory Monitoring Inc., Brown Deer, WI, USA). Oxyhaemoglobin saturation was monitored using pulse oximetry (OLV-3100; Nihon Kohden, Kobe, Japan). Tracheal sound was recorded from an air-coupled microphone (ECM-PC60, Sony, Japan) attached to the neck over the trachea to quantify snoring. Each monitoring record was scored in 30-s epochs by dividing data into consecutive segments of 30-s length. A single sleep or awake stage out of 5 different stages (sleep stages N1, N2, N3, rapid eye movement (REM) and awake) was assigned to each epoch based on the scoring manual of American Academy of Sleep Medicine (AASM) (19)
All 6 subjects discontinued antihistamines for 3 days prior to the PSG test. Scratching was measured in all subjects using Itch Tracker simultaneously with PSG. If 1 epoch contained scratching and the previous epoch did not, the previous epoch was considered as the pre-scratching epoch. If 1 epoch contained scratching and the next epoch did not, the next epoch was considered as the post-scratching epoch. Pre- and post-scratching epochs were compared in order to investigate the change in sleep stages after scratching. Multiple sets of scratching were considered as 1 episode of scratching if they occurred one after another without intervals of 1 full epoch or longer (Fig. 1).
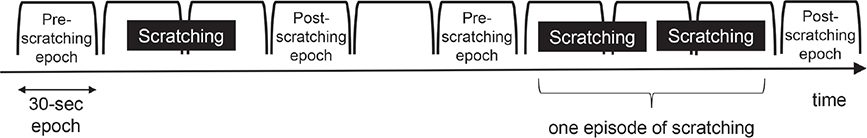
Fig. 1. Examples of pre- and post-scratching epochs in the analysis of polysomnography (PSG) data. The PSG record was divided into consecutive epochs 30-s long. The data analysis determined the sleep stage in each epoch. If 1 epoch contained scratching and the previous epoch did not, the previous epoch was considered as the pre-scratching epoch. If 1 epoch contained scratching and the next epoch did not, the next epoch was considered as the post-scratching epoch. Pre- and post-scratching epochs were compared in order to investigate the change in sleep stages after scratching. Multiple sets of scratching were considered as 1 episode of scratching if they occurred one after another without intervals of 1 full epoch or longer.
The study also evaluated sleep efficiency, the Wake-time After Sleep Onset (WASO), the total number of scratching episodes, and the total scratching time. Sleep efficiency (%) was defined as the total sleep time, which was the total time of different sleep stages (N1, N2, N3 and REM), divided by the total test time from bedtime to waking up.
For statistical analysis, the correlation of EASI vs scratching time/number as well as POEM vs EASI and scratching time/number was tested by Spearman’s correlation coefficient (Stata ver. 14.2., StataCorp LLC, College Station, TX, USA). Descriptive analysis was conducted for the other data.
RESULTS
Part 1 included 14 patients. There was a statistically significant positive correlation between EASI and the number of scratching episodes per h (r = 0.70, p = 0.0049) as well as between EASI and the total scratching time per h (r = 0.72, p = 0.0037) (Fig. 2a and 2b). In all cases except for the one with the highest EASI score, there was an over 30-min blank time that passed without scratching. In the case with the highest EASI score, scratching was detected constantly through the night. The results of POEM were obtained in 12 cases, all patients and parents answered that it was itchy every day in the answer to Question 1 of POEM. There was no statistically-significant correlation of POEM scores vs EASI scores (r = 0.38, p = 0.20) (Fig. 2c), the number of scratching episodes per h (r = 0.36, p = 0.23) or the total scratching time per h (r = 0.41, p = 0.16). Likewise, there was no significant correlation between POEM scores and the number of night scratching or the total scratching time (data not shown).
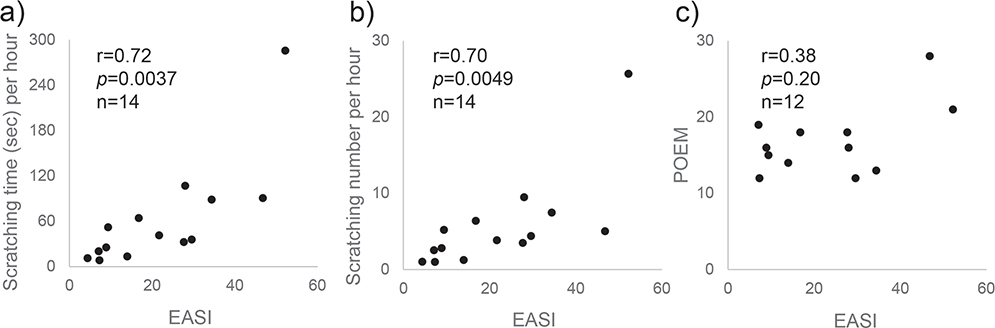
Fig. 2. Correlation of Eczema Area and Severity Index (EASI) scores with scratching time/number and Patient-Oriented Eczema Measure (POEM) scores. There was a statistically-significant positive correlation between (a) EASI and the number of scratching episodes per h (r = 0.70, p = 0.0049) as well as between (b) EASI and the total scratching time per h (r = 0.72, p = 0.0037), whereas (c) there was no statistically-significant correlation of POEM scores vs EASI scores (r = 0.38, p = 0.20).
Part 2 included 6 patients. Although the number of cases followed up to the second or third visit was too small to perform statistical analysis, there was a tendency of positive correlation in the change in EASI, total scratching time per h, and POEM (Fig. 3).
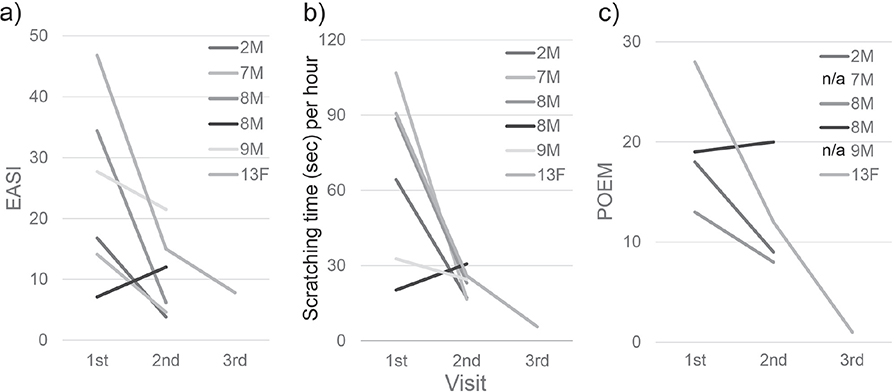
Fig. 3. Change in Eczema Area and Severity Index (EASI), scratching time per hour and Patient-Oriented Eczema Measure (POEM) after standard treatment. There was a tendency of positive correlation in the change of (a) EASI, (b) scratching time per h, and (c) POEM.
Part 3 included 6 patients. They had an EASI score of 13.7 ± 3.4 (meanvSD) and a sleep efficiency of 90.0 ± 4.4% (mean ± SD) (Table I). Although all of the 6 subjects had AD of moderate severity at the time of the test, 2 of them (a 10-year-old girl and a 14-year-old boy) had a standard medical treatment for AD for the first time within 1 week, and the severity of their eczema and itching was improving rapidly (Improving cases). The other 4 subjects were in the persistently moderate severity of AD for years despite the standard treatment (Persistent cases). The sleep efficiency had no tendency of correlation with EASI scores, but, instead, was relatively high in the 2 Improving cases (95.4% and 93.5%) compared with the 4 Persistent cases (92.1%, 88.7%, 85.7% and 84.6%) (Table I). There was a tendency to negative correlation between sleep efficiency and total number of scratching episodes.
As for the ratio of different sleep stages out of the total sleep time, N1 was 5.8 ± 2.0%, N2 was 44.1 ± 8.2%, N3 was 29.0 ± 6.5%, and REM was 21.1 ± 3.7% (mean ± SD in 6 subjects). In a total of 67 episodes of scratching, the pre-scratching epochs were awake in 49.3%, N1 in 9.0%, N2 in 28.4%, N3 in 10.4%, and REM in 3.0% (Fig. 4). In the 34 episodes of scratching that had a pre-scratching epoch of any sleep stage, i.e. N1, N2, N3 or REM, the sleep stage in the pre-scratching epoch was either N1 or N2 (light sleep) in 73.5%, whereas N3 (deep sleep) in 20.6%. In the same 34 episodes of scratching, the sleep stage was unchanged from the pre-scratching epoch to the post-scratching epoch in 58.8%, whereas the sleep stage became deeper in 5.9% and the sleep stage became lighter or changed to the awake stage in 35.3% (became lighter in 11.8%, changed to awake stage in 23.5%) (Fig. 5).
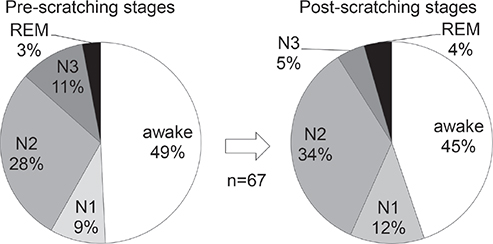
Fig. 4. Sleep and awake stages in pre- and post-scratching epochs. In a total of 67 episodes of scratching, the most frequent stage was awake, nearly 50%, which was followed by sleep stage N2, in both pre- and post-scratching epochs. REM: rapid eye movement.
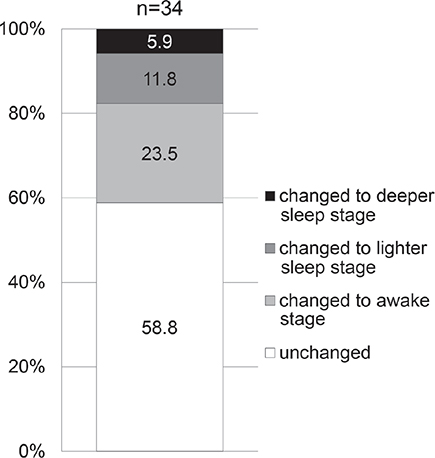
Fig. 5. Change in sleep stages after scratching. In the 34 episodes of scratching that had a pre-scratching epoch of any sleep stage, i.e. N1, N2, N3 or rapid eye movement (REM), the sleep stage became lighter or changed to the awake stage in 35.3% (became lighter in 11.8%, changed to awake stage in 23.5%) of episodes.
The total number of scratching episodes that had a pre-scratching epoch of any sleep stage, i.e. N1, N2, N3 or REM, was higher in the Persistent cases compared with the Improving cases (7.5 vs 2.0, mean). In the 2 Improving cases, the sleep stage was always unchanged after scratching. On the other hand, in the 30 episodes of scratching in the 4 Persistent cases, the sleep stage was unchanged in 53.3%, but became lighter or was changed to the awake stage in 40.0%.
DISCUSSION
The mechanism of sleep disorders in children with AD has not been clarified. It is known that itching worsens at night and might be related to sleep disorders (24). The current study results show that the higher the severity of AD, the longer the nocturnal scratching tends to be.
Actigraphy has been used in some previous studies to investigate sleep disturbance in adult and paediatric patients with AD by measuring body movement during sleep (13–17). It has also been demonstrated that the nocturnal scratching time estimated from actigraphy’s data correlates with the severity of AD (17). However, actigraphy potentially underestimates sleep time, since it is difficult for actigraphy to differentiate between scratching and other types of body movement, and long scratching could be considered as awakening, even if patients scratch while sleeping without awakening. To overcome this problem, the study used Itch Tracker to measure nocturnal scratching and PSG to evaluate sleep disturbance. Itch Tracker has a unique algorithm to detect scratching behaviour and it has been demonstrated to have high sensitivity and positive predictive values in adult patients with AD (12).
Part 1 found that EASI scores correlated significantly with nocturnal scratching time and number of scratching episodes. Although EASI is a useful tool for assessing the severity of AD, EASI scores must be carefully compared between different individuals with AD, because the results may vary depending on the assessing physicians. In this study, the same 2 physicians, both of whom have expertise in paediatric dermatology, always evaluated EASI together to avoid variation. The demonstrated correlation of EASI scores with nocturnal scratching time and number was similar to the previous study with Itch Tracker in adult patients with AD (12).
On the other hand, POEM scores did not significantly correlate with EASI scores nor with nocturnal scratching time/number. The result, that there was no significant correlation between POEM and EASI scores, is not surprising and is similar to a previous report in children with AD (25). Interestingly, all 12 cases had the same answer of “itching every day in the previous week” to Question 1 for the POEM, regardless of the severity of their AD. This indicates that the subjective evaluation of itch frequency is not sensitive enough to the severity of itch in patients.
The results of Part 2 showed that the nocturnal scratching time became shorter as the symptoms of AD (EASI and POEM) improved. In contrast, the nocturnal scratching time became longer in the 1 case in which the symptoms of AD worsened. This result indicates that the change in nocturnal scratching time is a good index reflecting the therapeutic efficacy in children with AD, similarly to the change in EASI and POEM scores. Although POEM scores are known to correlate well with the severity of AD in adult patients (26), the nocturnal scratching time would be a more useful index than POEM in children, as children are less capable and reliable in answering the POEM questions.
In Part 3 the sleep efficiency was 84.6% or higher in all of the 6 cases examined (mean ± SD; 90.0 ± 4.4%). This result is similar to the previously reported studies in which sleep efficiency was evaluated by PSG in children with AD (27–29). The sleep efficiency tends to be lower when it was measured by actigraphy compared with PSG (28). Although it was reported that children with AD had significantly reduced sleep efficiency, longer sleep onset latency, more sleep fragmentations, and less non-rapid eye movement (non-REM) sleep compared with healthy controls (28), the current study had no healthy controls and hence could not confirm these findings.
Previous reports on scratching during sleep found that scratching occurred mainly during light sleep stages, i.e. N1 and N2, and sporadically at the onset of sleep (28–30). The current results were similar to this, showing that the sleep stages in which scratching started were light stages, rather than deep stages. It remains to be clarified whether itching changes the sleep stage to a lighter one or itching is likely to be felt when the sleep stage is light.
It has been reported in a previous study using PSG focusing on the relationship between AD and quality of sleep in adult patients that the sleep stage often changed to a lighter stage after scratching and that deep sleep stages, i.e. N3 and N4, were shortened (30). In the current study, the sleep stage changed to a lighter one or the awake stage in 35.3% of cases. This indicates that quality of sleep in children with AD is impaired due to scratching just as in adults. Moreover, the results of the current study show that the sleep stage changed to a lighter one or the awake stage after 40.0% of scratching episodes in children whose dermatitis was persistent, whereas the sleep stage never changed after scratching and the sleep efficiency was relatively high in children whose dermatitis was improving even though the EASI score was high and the total scratching time was long at the time, as in the case of a 10-year-old girl. Although this finding is based on a small number of cases, this might indicate that the impairment of quality of sleep due to scratching in patients with AD is not only linked to the absolute severity of dermatitis, but also to the direction of change in the severity of dermatitis, i.e. whether the severity of dermatitis is improving, persistent, or worsening.
This study has some limitations. First, there was no healthy control group. Secondly, self-subjectivity and parental subjectivity were inevitably mixed in POEM scores. Since PSG was conducted only during 1 overnight for each subject, it is expected that the subjects were not accustomed to the examination environment. Moreover, since PSG was conducted only for a small number of children with moderate AD, a further study with a larger number of children with both moderate and severe AD will be needed to verify the assumption obtained from this study. Even with these limitations, however, this study clearly demonstrates that the severity of AD correlates with nocturnal scratching time, and that nocturnal scratching can result in deterioration of quality of sleep by changing the sleep stage to a lighter one or to the awake stage.
This is the first study to assess nocturnal scratching and evaluate its relationship with depth of sleep in children with AD using PSG and Itch Tracker at the same time. The results support that Itch Tracker is as applicable for children as for adults to measure nocturnal scratching. Since nocturnal scratching is an important factor in deterioration of quality of sleep, as has demonstrated in this study, Itch Tracker is expected to be a useful tool for the measurement of quality of sleep in patients with chronic pruritus.
ACKNOWLEDGEMENTS
The authors thank the paediatric outpatient co-medical workers involved in this study, especially nurse Nao Ikeda, for taking time to explain the study to the patients.
This study was internally funded by the National Hospital Organization, Fukuoka National Hospital.
IRB approval status: This study was approved by the ethics committee of Fukuoka National Hospital (approval number F30-21). Parental consent was obtained for all subjects. Subjects aged 6 years or older provided informed consent for participation in writing.
REFERENCES
- Fishbein AB, Vitaterna O, Haugh IM, Bavishi AA, Zee PC, Turek FW, et al. Nocturnal eczema: review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol 2015; 136: 1170–1177.
- Thorburn PT, Renata L Riha. Skin disorders and sleep in adults: where is the evidence? Sleep Med Rev 2010; 14: 351–358.
- Ebata T, Aizawa H, Kamide R, Niimura M. The characteristics of nocturnal scratching in adults with atopic dermatitis. Br J Dermatol 1999; 141: 82–86.
- Yano C, Saeki H, Ishiji T, Ishiuji Y, Sato J, Tofuku Y, et al. Impact of disease severity on sleep quality in Japanese patients with atopic dermatitis. J Dermatol Sci 2013; 72: 195–197.
- Li JC, Fishbein A, Singam V, Patel KR, Zee PC, Attarian H, et al. Sleep disturbance and sleep-related impairment in adults with atopic dermatitis: a cross-sectional study. Dermatitis 2018; 29: 270–277.
- Arima K, Gupta S, Gadkari A, Hiragun T, Kono T, Katayama I, et al. Burden of atopic dermatitis in Japanese adults: analysis of data from the 2013 National Health and Wellness Survey. J Dermatol 2018; 45: 390–396.
- Schmitt J, Romanos M, Schmitt NM, Meurer M, Kirch W. Atopic eczema and attention-deficit/hyperactivity disorder in a population-based sample of children and adolescents. JAMA 2009; 301: 724–726.
- Strom MA, Fishbein AB, Paller AS, Silverberg JI. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol 2016; 175: 920–929.
- Horev A, Freud T, Manor I, Cohen AD, Zvulunov A. Risk of attention-deficit/hyperactivity disorder in children with atopic dermatitis. Acta Dermatovenerol Croat 2017; 25: 210–214.
- Romanos M, Gerlach M, Warnke A, Schmitt J. Assotiation of attention-deficit/hyperactivity disorder and atopic eczema modified by sleep disturbance in a large population-based sample. J Epidemiol Community Health 2010; 64: 269–273.
- Ebata T. Measurement of itch: actigraphy. In: Misery L, Staender S, editors. Pruritus. Second edition. Springer-Verlag, London, UK; 2016: p. 97–101.
- Ikoma A, Ebata T, Chantalat L, Takemura K, Mizzi F, Poncet M, et al. Measurement of nocturnal scratching in patients with pruritus using a smartwatch: initial clinical studies with the Itch Tracker App. Acta Derm Venereol 2019; 99: 268–273.
- Bender BG, Leung SB, Leung DY. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. J Allergy Clin Immunol 2003; 111: 598–602.
- Sandoval LF, Huang K, O’Neill JL, Gustafson CJ, Hix E, Harrison J, et al. Measure of atopic dermatitis disease severity using actigraphy. J Cutan Med Surg 2014; 18: 49–55.
- Gustafson CJ, O’Neill J, Hix E, McLaren DT, Buxton OM, Feldman SR. Feasibility of actigraphy wristband monitoring of atopic dermatitis in children. Skin Res Technol 2014; 20: 510–514.
- Ebata T, Iwasaki S, Kamide R, Niimura M. Use of a wrist activity monitor for the measurement of nocturnal scratching in patients with atopic dermatitis. Br J Dermatol 2001; 144: 305–309.
- Fujita H, Nagashima M, Takeshita Y, Aihara M. Correlation between nocturnal scratch behavior assessed by actigraphy and subjective/objective parameters in patients with atopic dermatitis. Eur J Dermatol 2014; 24: 120–122.
- Kolla BP, Mansukhani S, Mansukhani MP. Consumer sleep tracking devices: a review of mechanisms, validity and utility. Expert Rev Med Devices 2016; 13: 497–506.
- Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, at et al. American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.3. www.aasmnet.org. Darien, IL: American Academy of Sleep Medicine, 2016.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004; 140: 1513–1519.
- Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013; 169: 1326–1332.
- Spuls PI, Gerbens LAA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol 2017; 17: 979–984.
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. Share. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012; 67: 99–106.
- Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol 2007; 87: 295–298.
- Ridd MJ, Gaunt DM, Guy RH, Redmond NM, Garfield K, Hollinghurst S, et al. Comparison of patient (POEM), observer (EASI, SASSAD, TIS) and corneometry measures of emollient effectiveness in children with eczema: findings from the COMET feasibility trial. Br J Dermatol 2018; 179: 362–370.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, Ulzii D, Furue M. Patient-Oriented Eczema Measure score: a useful tool for web-based surveys in patients with atopic. Acta Derm Venereol 2020; 100: adv00159.
- Chang YS, Chou YT, Lee JH, Dai YS, Sun C, Lin YT, et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics 2014; 134: e397–405.
- Reuveni H, Chapnick G, Tal A, Tarasiuk A. Sleep fragmentation in children with atopic dermatitis. Arch Pediatr Adolesc Med 1999; 153: 249–253.
- Gillow G, Jackson K, Mukherji J, Buranosky B, Palomo R, Sheldon S, et al. Timing of scratch and limb movements during sleep in children with atopic dermatitis. Pediatr Dermatol 2023; 40: 305–307.
- Aoki T, Kushimoto H, Hishikawa Y, Savin JA. Nocturnal scratching and its relationship to the disturbed sleep of itchy subjects. Clin Exp Dermatol 1991; 16: 268–272.