ORIGINAL REPORT
Response to Interleukin-17A Inhibitors According to Prior Biologic Exposures: A Danish Nationwide Study
Nikolai LOFT1,2, Alexander EGEBERG3,4, Daniel ISUFI1,2, Mads K. RASMUSSEN5, Lars E. BRYLD6, Tomas N. DAM7, Kawa K. AJGEIY8, Trine BERTELSEN5 and Lone SKOV1,2,4
1Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, 2Copenhagen Research Group for Inflammatory Skin, Herlev and Gentofte Hospital, Hellerup, 3Department of Dermatology, Bispebjerg Hospital, 4Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, 5Department of Dermatology, Aarhus University Hospital, Aarhus, 6Department of Dermatology, Zealand University Hospital, Roskilde, 7Dermatology Clinic, Nykoebing Falster and 8Department of Dermatology, Odense University Hospital, Odense, Denmark
Whether response to an interleukin (IL-17) inhibitor is different in patients with previous exposure to an IL-17 inhibitor compared with patients with exposure to biologics with other cytokine targets remains to be elucidated. Therefore, the aim of this study was to assess whether previous exposure to an IL-17A inhibitor was associated with worse response than exposure to (an)other biologic(s). All patients in the DERMBIO register treated with an IL-17A inhibitor (secukinumab or ixekizumab) were included. With an absolute Psoriasis Area and Severity Index (PASI) ≤ 2 as response, the proportion of responders treated with IL-17A inhibitors was assessed in patients previously treated with another IL-17A inhibitor and compared with patients with previous exposure to (an)other biologic(s), using a χ2 test. In total, 100, 93 and 83 patients with previous exposure to an IL-17A inhibitor and 414, 372 and 314 patients with previous exposure to (an) other biologic(s) were assessed after 3, 6 and 12 months, respectively. No differences in the proportion of patients achieving PASI ≤ 2 were observed between the 2 groups after 3 months (54% vs 57%, p = 0.59), 6 months (70% vs 66%, p = 0.42) and 12 months (69% vs 60%, p = 0.14). In conclusion, when treating patients with IL-17A inhibitors the cytokine target of the previous biologic does not appear to affect the response.
Key words: psoriasis; biologics; IL-17; secukinumab; ixekizumab; switch.
SIGNIFICANCE
This study examined whether patients who were previously treated with an interleukin-17A inhibitor responded differently to another interleukin-17A inhibitor compared with those who had been treated with other biologics. The study included patients treated with secukinumab or ixekizumab. After 3, 6 and 12 months, no significant difference was found in the proportion of patients achieving a good response (Psoriasis Area and Severity Index ≤ 2) between those with prior exposure to interleukin-17A inhibitor and those with exposure to other biologics. In conclusion, prior exposure to an interleukin-17A inhibitor does not appear to affect the response when treating patients with interleukin-17A inhibitors.
Citation: Acta Derm Venereol 2023; 103: adv12616. DOI https://doi.org/10.2340/actadv.v103.12616.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Oct 19, 2023; Published: Nov 21, 2023
Corr: Nikolai Loft, Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Hellerup, Denmark. E-mail: nikolai.nguyen.loft@regionh.dk
INTRODUCTION
Based on data from clinical trials, interleukin (IL)-17 inhibitors are among the biologics for treating moderate-to-severe psoriasis with the best efficacy and show a good safety profile (1–3). Patients in the real-world have more comorbidities and have often received more treatments than those participating in clinical trials (4). In addition, patients who have previously been treated with biologics with a similar cytokine target as the investigated biologics have often been prohibited from participating in clinical trials. In general, real-world studies report lower responses in those with previous biologic exposure compared with biologic-naïve patients for IL-17 inhibitors (5–11). Whether the cytokine target of previous biologics affects the response to the current biologic has not been well-investigated, and as multiple biologics target the IL-17 cytokine family, it is important to assess whether a class effect exists, e.g. failure of one IL-17 inhibitor leads to failure of another. Few smaller studies have indicated that previous treatment with one IL-17 inhibitor does not appear to affect the efficacy of another IL-17 inhibitor (12–23). However, these studies often lack information on the reasons for discontinuation of previous anti-IL-17 treatment (12). In addition, whether response to an IL-17 inhibitor is different in patients with previous exposure to an IL-17 inhibitor compared with exposure to (an)other cytokine target(s) remains to be elucidated.
This study examined the effectiveness of IL-17A inhibitors (secukinumab and ixekizumab) in a real-world nationwide setting according to cytokine targets of prior exposure to biologics.
MATERIALS AND METHODS
The study was approved by the Danish Data Protection Agency (ref. VD-2018-286). In Denmark, approval of an ethics committee is not required for register-based studies.
Patient selection
All patients in DERMBIO (24–34) with a treatment series with an IL-17A inhibitor (secukinumab or ixekizumab) between April 2015 and October 2019 were eligible for inclusion.
Patients were categorized into 2 groups according to biologic exposure: (i) patients with previous IL-17A inhibitor exposure (secukinumab or ixekizumab); and (ii) patients with biologic exposure other than IL-17 inhibitors. Patients with previous exposure to an IL-17A inhibitor were grouped according to the reason for discontinuation of previous IL-17A inhibitor: treatment failure vs reasons other than treatment failure. Treatment failure was defined as primary failure or secondary failure. Primary failure was defined as failure to achieve Psoriasis Area and Severity Index (PASI) ≤ 4 or PASI75 after 3 months, or discontinuation of treatment before or at the 3 months visit due to lack of efficacy. Secondary failure was defined as achieving PASI ≤ 4 and/or PASI75 after 3 months and stopping treatment afterwards due to loss of effectiveness, or for patients without a recorded PASI after 3 months of treatment, stopping treatment beyond the 6-month visit due to lack/loss of efficacy.
Outcomes
The effectiveness of the IL-17A inhibitors was assessed by response criteria based on absolute PASI comparing patients with previous IL-17 inhibitor exposure with patients with biologics exposure other than IL-17 inhibitors. The primary outcome was an absolute PASI ≤ 2 after 3, 6 and 12 months, respectively. Secondary outcomes were the proportion of patients achieving PASI = 0 and PASI ≤ 4 after 3, 6 and 12 months, respectively. Tertiary outcomes were based on relative reductions of PASI; PASI75, PASI90 and PASI100. Lastly, the proportion of patients achieving DLQI = 0/1 was assessed.
Responder imputation
As the data originate from the real-world and patients might have missing data/visits due to multiple reasons, including lack of effectiveness, lack of registration, visits at other times than scheduled, etc., different methods were used to analyse data. The primary model was constructed using a modified last observation carried forward (LOCF) approach: patients who stopped treatment prior to the evaluation point (month 3, 6 and 12) due to lack of effectiveness were considered non-responders, for the rest of the patients the last observation was carried forward to the evaluation point and the response was based on this value.
As subanalyses and for meta-data the following approaches were used: as observed (AO), LOCF, non-responder imputation (NRI), and a modified AO approach, where patients who stopped treatment prior to the evaluation point (month 3, 6 and 12) due to lack of effectiveness were considered non-responders and the rest of patients were analysed AO.
For all models, only patients with long enough follow-up were considered, e.g. only patients starting treatment 3, 6 and 12 months before database lock, were considered for analysis at the relevant time-points.
Statistical analysis
For continuous data, means with standard deviations (SD) and medians with interquartile ranges (IQR) were reported, and for categorical outcomes, numbers with percentages were presented. For normally distributed data an unpaired t-test was used to assess differences between groups, and for non-parametric data Mann–Whitney test was used. For categorical outcomesχ2 or Fisher’s exact tests were used to assess differences between groups.
The effectiveness of IL-17A inhibitors was assessed and compared in different groups: (i) patients with previous exposure to IL-17A inhibitors vs patients with previous exposure to (an)other biologic(s) than IL-17 inhibitors; (ii) patients with treatment failure to previous IL-17A inhibitors vs patients who stopped treatment due to other reasons than treatment failure of previous IL-17A inhibitor; (iii) primary failure vs secondary failure to previous IL-17A inhibitor. The outcomes were assessed for IL-17A inhibitors overall and for the individual drug.
The association between previous exposure to biologics and response to drug was assessed for the primary outcome (PASI ≤ 2 after 3, 6 and 12 with a modified-LOCF approach) in a sensitivity analysis using logistic regression. The odds ratios (ORs) are presented as crude, partially adjusted and fully adjusted. The partially adjusted model included drug, and the fully adjusted model included additional adjustment for sex, age, weight and number of previous therapies (1 vs > 1).
All data management and statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Baseline characteristics
In total, 581 treatment series with an IL-17A inhibitor were included, of which 151 series were with ixekizumab and 430 treatment series with secukinumab. Of the included patients, 121 were previously exposed to another IL-17A inhibitor, and 460 had previous exposure to (an)other biologic(s) than an IL-17 inhibitor (Table I). Most of the patients with previous exposure to IL-17A inhibitors were treated with ixekizumab and were previously exposed to secukinumab, whereas most patients exposed to a biologic with another mechanism of action were treated with secukinumab (Table I). Patients previously exposed to an IL-17A inhibitor had been treated with more biologics than those with previous biologic exposure but naïve to IL-17 inhibitors (Table I).
Effect of interleukin-17A inhibitors in previously biologic- exposed patients
Of the included patients, 556 had follow-up time of minimum 3 months, 486 of minimum 6 months and 402 of minimum 12 months (Fig. 1). The overall proportion of patients treated with an IL-17A inhibitor who achieved PASI ≤ 2 was respectively, 56% after 3 months, 66% after 6 months and 62% after 12 months using the modified LOCF approach.
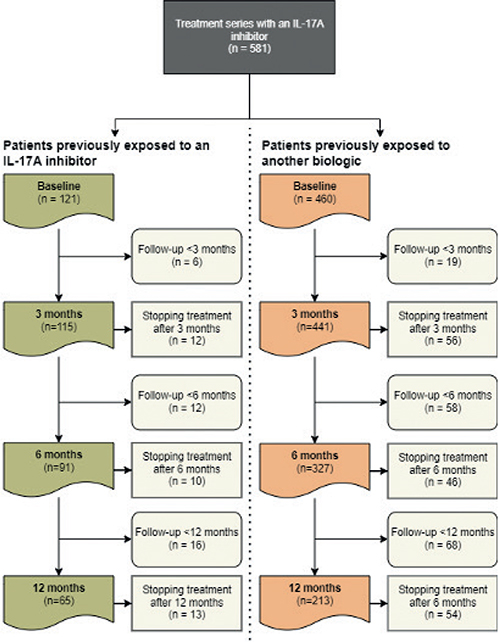
Fig. 1. Flowchart showing number of patients available for analysis at different time points IL: interleukin.
Patients with previous exposure to interleukin-17A inhibitor vs biologics other than interleukin-17 inhibitors
Of the included patients, 115, 103 and 87 with previous exposure to an IL-17A inhibitor had follow-up time of minimum 3, 6 and 12 months, respectively; and 441, 383 and 315 with previous exposure to (an)other biologic(s) than an IL-17 inhibitor had follow-up time of minimum 3, 6 and 12 months, respectively (Fig. 1). The number of patients that could be analysed using modified LOCF was 100, 93 and 83 for patients with previous exposure to an IL-17A inhibitor and 414, 372 and 314 for patients with previous exposure to an(other) biologic(s) than an IL-17 inhibitor after 3, 6 and 12 months respectively. No differences in the proportion of patients achieving PASI ≤ 2 was observed after 3 months (n: 54 [54%] vs n: 236 [57%], p = 0.59), 6 months (n: 65 [70%] vs n: 244 [66%], p = 0.42), and 12 months (n: 56 [69%] vs 189 [60%], p = 0.14), respectively (Fig. 2). Similar results were observed for all other outcomes (Table SI). When analysing data using NRI, it appeared that a higher proportion of patients responded to IL-17A inhibitors among those exposed to (an)other biologic(s) than an IL-17 inhibitor after 3 and 12 months for most outcomes (Table SI). This was not observed after 6 months or for any of the other responder imputations (Table SI).
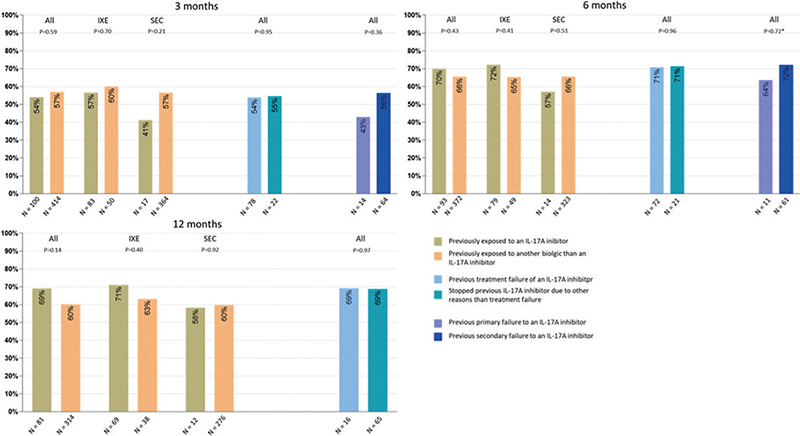
Fig. 2. Response in patients treated with an interleukin (IL)-17A inhibitor. Proportion of patients achieving a Psoriasis Area and Severity Index (PASI) ≤ 2 after: (a) 3 months, (b) 6 months, and (c) 12 months, using a modified last observation carried forward (LOCF) approach. Patients are analysed according to previous biologic exposure. IXE: ixekizumab; SEC: secukinumab. *Fisher’s exact test.
Reasons for discontinuation of previous interleukin-17A inhibitor: treatment failure vs other reasons than treatment failure
Of the patients with previous exposure to an IL-17A inhibitor, 93 stopped the previous IL-17A inhibitor due to treatment failure and 28 stopped due to other reasons. Overall, with the modified LOCF approach, 100 patients could be analysed after 3 months, 93 after 6 months and 81 after 12 months. No differences in the proportion of patients achieving PASI ≤ 2 after 3 months (54% vs 55%, p = 0.95), 6 months (71% vs 71%, p = 0.96), and 12 months (69% vs 69%, p = 0.97) were observed between the 2 groups (Fig. 1). Similar results were observed for all other outcomes although the proportion of patients achieving PASI ≤ 4 appeared to be higher in those with previous treatment failure at all time-points (Table SII). Changing the responder imputation did not alter the results (Table SII).
Reasons for previous treatment failure IL-17A inhibitor: primary failure vs secondary failure
In total, 18 patients stopped treatment of previous IL-17A inhibitors due to primary failure and 75 stopped due to secondary failure. Overall, with the modified LOCF approach, 78 patients could be analysed after 3 months and 72 after 6 months. Response rates could not be assessed after 12 months due to the limited number of patients. Throughout the treatment cause, no statistically significant difference was observed in the proportion of patients achieving PASI ≤ 2 (Fig. 2). However, more patients appeared to achieve PASI ≤ 2 (43% vs 56%, p = 0.36) and PASI ≤ 4 (57% vs 75%, p = 0.18) after 3 months in patients with previous secondary failure, although this was not statistically significant and was not observed after 6 months (Table SIII).
Sensitivity analyses
Sensitivity analyses were performed for PASI ≤ 2 using a modified LOCF approach after 3, 6 and 12 months. Here, no association between previous exposure to biologics (IL-17 vs (an)other biologic(s)) and response to treatment with an IL-17A inhibitor was observed (Table SIV). Similarly, no association was observed between the previous reason for drug discontinuation (failure vs other reasons than failure) or for treatment failure (primary vs secondary failure) and response to treatment with an IL-17A inhibitor (Table SIV). This was observed for the crude analyses and for the adjusted analysis. Of the explaining variables only body weight seemed to influence the response (data not shown).
DISCUSSION
This nationwide register-based study of 581 patients treated with IL-17A inhibitors with previous biologic exposure, observed that 56%, 66% and 62% of the patients achieved PASI2 after 3, 6 and 12 months using a modified LOCF imputation. No differences were found in response regarding the cytokine target of the previous biologic or the reason for discontinuation of the previous IL-17A inhibitor.
In phase 3 clinical trials, the proportion of patients with previous exposure to biologics achieving PASI75, PASI90 and PASI100 were 88%, 68% and 37% after 3 months in patients with psoriasis treated with labelled doses of ixekizumab (35). The corresponding proportion of patients with PASI75, PASI90 and PASI100 in real-world studies are generally reported to be similar or lower (5, 8, 18). However, clinical trials and real-world studies differ in several settings. The follow-up time in real-world studies is often incomplete and handling of patients with incomplete follow-up time is not always clear. Furthermore, patients are seen more often and at specific time-points in clinical trials. Thus, missing values in clinical trials more likely represent a non-responder; consequently, non-responder imputation is often opted for in clinical trials. On the other hand, in the real-world, patients might be seen at other time-points than the scheduled times, registration is not as vigilant as in clinical trials, and missing values do not necessarily represent a non-responder. Non-responder imputation might therefore be too conservative, whereas imputation as observed does not take patients stopping treatment prior to evaluation into account. Both NRI and AO imputation have been used in real-world studies, but which approach is being used is not always clear (8, 13–19, 36). In the current study, the main outcome was based on a modified LOCF imputation as patients stopping treatment due to lack of effect were also considered and, by doing so, the highest possible number of patients could be included. In addition, this study presents all other responder imputation, which did not alter the results. In addition, the study used response criteria based on absolute PASI, as these are more suitable in real-world, considering the lack of wash-out of prior treatment (37). An absolute PASI ≤ 2 as response criterion appears to be optimal (38) and has been shown to correspond to PASI90 (37, 39). With a modified LOCF imputation, 56% of all patients with previous exposure to biologics regardless of mechanism of action achieved PASI ≤ 2 after 3 months of treatment with an IL-17A inhibitor, which is lower than the proportion of patients with previous biologic exposure who achieve PASI90 in phase III trials (35). However, biologics with similar mechanism of action as the investigated biologics are often prohibited in clinical trials and patients in the real-world who are ineligible for clinical trials often have worse response than those who are eligible (40). A recent systematic review and meta-analysis showed that previous exposure to an IL-17 inhibitor did not necessarily predict failure to a subsequent IL-17 inhibitor (12). The meta-analysis could not assess whether the reason for discontinuation of the previous IL-17 inhibitor influenced response or if previous exposure to an IL-17 inhibitor was associated with worse response than exposure to another biologic with a different mechanism of action (12). In the current study, the cytokine target of previous biologics did not appear to influence the response. In addition, for patients with previous exposure to an IL-17A inhibitor, the reason for discontinuation of a previous IL-17A inhibitor did not affect the response. This indicates that there is no class effect regarding failure to respond to IL-17A inhibitors. However, after 3 months it appeared that more patients with previous secondary failure responded to an IL-17A inhibitor compared with patients with previous primary failure. Nevertheless, only a few patients with previous primary treatment switched to another IL-17 inhibitor, and the difference was not statistically significant.
Some limitations should be considered; due to limited data it was not possible to differentiate ixekizumab and secukinumab for some of the exposures. The lack of difference in response among patients with previous exposure to different cytokine targets could be due to the limited number of patients included. However, all patients in Denmark treated with an IL-17A inhibitor were included and any differences would be considered of little clinical relevance. Another limitation is the missing data on effect measures. Still, this study presents different approaches to analysing data and considers data to be missing at random between the groups. Finally, it is possible that factors other than previous treatment might have influenced the outcome; however, sensitivity analyses including multiple possible confounding variables were performed, which did not influence the results. The strengths of the study include the nationwide coverage of patients, the validated disease measures and response criteria, and the prospective collection of data.
In conclusion, when treating patients with IL-17A inhibitors the cytokine target of the previous biologic and the reason for discontinuation of a previous biologic do not appear to affect the response. However, additional studies assessing the individual IL-17A inhibitor are needed.
ACKNOWLEDGEMENTS
NL has been an honorary speaker for Eli Lilly, Janssen Cilag, and Sandoz. AE has received research funding from Pfizer, Eli Lilly, Novartis, AbbVie, Boehringer Ingelheim, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Galapagos NV, Sun Pharmaceuticals, Leo Pharma, Samsung Bioepis Co., Ltd, Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, Union Therapeutics, and Janssen Pharmaceuticals. MKR has been a paid speaker for AbbVie, Almirall, and LEO Pharma. Consulting or serving on expert/advisory boards with AbbVie, Almirall, Janssen Cilag, and Eli Lilly. Served as investigator for Janssen Cilag, and Novartis. LEB has received educational grants from Janssen Pharmaceuticals and LEO Pharma. TND has been a paid speaker for Janssen Cilag and has been a consultant or has served on Advisory Boards with AbbVie, Janssen Cilag, Novartis, Eli Lilly and LEO Pharma. TB has received research funding from Novartis, the Danish National Psoriasis Foundation, The A.P Moller foundation, Wehnerts Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and received educational grants from Pfizer, Novartis and Abbvie and been a paid speaker for Eli Lilly and Leo Pharma. LS has been a paid speaker for AbbVie, Eli Lilly, Novartis, Pfizer, and LEO Pharma, and has been a consultant or has served on Advisory Boards with AbbVie, Janssen Cilag, Novartis, Eli Lilly, Boehringer Ingelheim, LEO Pharma, UCB, Almirall, Bristol-Myers Squibb, and Sanofi. She has served as an investigator for AbbVie, Sanofi, Janssen Cilag, Boehringer Ingelheim, Eli Lilly, Novartis, Regeneron, and LEO Pharma, and has received research and educational grants/funding from LEO Foundation, Kgl Hofbundtmager Aage Bang Foundation, Novartis, Sanofi, Bristol-Myers Squibb, Janssen Cilag, Almirall and LEO Pharma. DI and KKA have no conflicts of interest to declare.
The DERMBIO registry has entered into agreements with Abbvie, Eli Lilly, Bristol-Myers Squibb, UCB, Novartis, Janssen, and Leo Pharma. They receive post-marketing data and have no influence on the data collection, statistical analyses, manuscript preparation or decision to submit.
REFERENCES
- Cui L, Chen R, Subedi S, Yu Q, Gong Y, Chen Z, et al. Efficacy and safety of biologics targeting IL-17 and IL-23 in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol 2018; 62: 46–58.
- Bilal J, Berlinberg A, Bhattacharjee S, Trost J, Riaz IB, Kurtzman DJ. A systematic review and meta-analysis of the efficacy and safety of the interleukin (IL)-12/23 and IL-17 inhibitors ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and tildrakizumab for the treatment of moderate to severe plaque psoriasis. J Dermatol Treat 2018; 29: 569–578.
- Loft N, Vaengebjerg S, Halling AS, Skov L, Egeberg A. Adverse events with IL-17 and IL-23 inhibitors for psoriasis and psoriatic arthritis: a systematic review and meta-analysis of phase III studies. J Eur Dermatol Venereol 2020; 34: 1151–1160.
- Garcia-Doval I, Carretero G, Vanaclocha F, Ferrandiz C, Daudén E, Sánchez-Carazo J-L, et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: patients ineligible vs eligible for randomized controlled trials. Arch Dermatol 2012; 148: 463–470.
- Ger T-Y, Huang Y-H, Hui RC-y, Tsai T-F, Chiu H-Y. Effectiveness and safety of secukinumab for psoriasis in real-world practice: analysis of subgroups stratified by prior biologic failure or reimbursement. Ther Adv Chronic Dis 2019; 10: 2040622319843756.
- Notario J, Deza G, Vilarrasa E, Valentí F, Muñoz C, Mollet J, et al. Treatment of patients with plaque psoriasis with secukinumab in a real-life setting: a 52-week, multicenter, retrospective study in Spain. J Dermatol Treat 2019; 30: 424–429.
- Galluzzo M, Talamonti M, De Simone C, D’Adamio S, Moretta G, Tambone S, et al. Secukinumab in moderate-to-severe plaque psoriasis: a multi-center, retrospective, real-life study up to 52 weeks observation. Expert Opin Biol Ther 2018; 18: 727–735.
- Chiricozzi A, Burlando M, Caldarola G, Conti A, Damiani G, De Simone C, et al. Ixekizumab effectiveness and safety in the treatment of moderate-to-severe plaque psoriasis: a multicenter, retrospective observational study. Am J Clin Dermatol 2020; 21: 441–447.
- Megna M, Di Costanzo L, Argenziano G, Balato A, Colasanti P, Cusano F, et al. Effectiveness and safety of secukinumab in Italian patients with psoriasis: an 84 week, multicenter, retrospective real-world study. Expert Opin Biol Ther 2019; 19: 855–861.
- Schwarz CW, Loft N, Rasmussen MK, Nissen CV, Dam TN, Ajgeiy KK, et al. Predictors of response to biologics in patients with moderate-to-severe psoriasis: a Danish nationwide cohort study. Acta Derm Venereol 2021; 101: adv00579.
- Loft N, Egeberg A, Rasmussen M, Bryld L, Nissen C, Dam T, et al. Prevalence and characterization of treatment-refractory psoriasis and super-responders to biologic treatment: a nationwide study. J Eur Acad Dermatol Venereol 2022; 36: 1284–1291.
- Loft N, Halling A-S, Egeberg A, Skov L. Efficacy of a second interleukin 17 inhibitor in patients with psoriasis: a systematic review and meta-analysis. J Am Acad Dermatol 2021; 84: 130–138.
- Bokor-Billmann T, Schäkel K. No need to change the drug class: ixekizumab-following secukinumab-therapy in psoriasis. J Dermatol Treat 2019; 30: 216–220.
- Khemis A, Kelati A, Montaudié H, Lacour J-P, Passeron T. Successful treatment of severe psoriasis relapse with secukinumab (interleukin 17 A inhibitor) after abrupt brodalumab (interleukin 17 receptor inhibitor) discontinuation: a retrospective study evaluating long-term efficacy and safety. J Am Acad Dermatol 2018; 79: 758–760.
- Georgakopoulos J, Phung M, Ighani A, Lam K, Yeung J. Biologic switching between interleukin 17A antagonists secukinumab and ixekizumab: a 12-week, multicenter, retrospective study. J Eur Acad Dermatol Venereol 2018; 33: e7–e8.
- Hegazy S, Konstantinou M, Bulai Livideanu C, Tauber M, Paul C. Efficacy of ixekizumab in patients with resistance or incomplete response to secukinumab. J Eur Acad Dermatol Venereol 2019; 33: e338–e341.
- Sherman S, Cohen ES, Amitay-Laish I, Hodak E, Pavlovsky L. IL-17A inhibitor switching–efficacy of ixekizumab following secukinumab failure. a single-center experience. Acta Derm Venereol 2019; 99: 769–773.
- Deza G, Notario J, Lopez-Ferrer A, Vilarrasa E, Ferran M, Del Alcazar E, et al. Initial results of ixekizumab efficacy and safety in real-world plaque psoriasis patients: a multicentre retrospective study. J Eur Acad Dermatol Venereol 2019; 33: 553–559.
- Amschler K, Phillip S, Mohr J, Wilsmann-Theis D, Poortinga S, Gerdes S, et al. Long-term follow-up of 22 psoriatic patients treated with ixekizumab after failure of secukinumab. Dermatol Online J 2020; 26: 13030/qt11d602x5.
- Enevold C, Loft N, Bregnhøj A, Zachariae C, Iversen L, Skov L, et al. Circulating brodalumab levels and therapy outcomes in patients with psoriasis treated with brodalumab: a case series. JAMA Dermatol 2022; 158: 762–769.
- Loft N, Bregnhøj A, Fage S, Nielsen CH, Enevold C, Zachariae C, et al. Effectiveness of brodalumab after previous treatment failure of interleukin-17A inhibitors in patients with psoriasis. Dermatol Ther 2021; 34: e15106.
- Bonifati C, Morrone A, Cristaudo A, Graceffa D. Effectiveness of anti-interleukin 23 biologic drugs in psoriasis patients who failed anti-interleukin 17 regimens. A real-life experience. Dermatol Ther 2021; 34: e14584.
- Sherman S, Zloczower O, Noyman Y, Amitay-Laish I, Hodak E, Pavlovsky L. Ixekizumab survival in heavily pretreated patients with psoriasis: a two-year single-centre retrospective study. Acta Derm Venererol 2020; 100: adv00349.
- Gniadecki R, Kragballe K, Dam T, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol 2011; 164: 1091–1096.
- Ahlehoff O, Skov L, Gislason G, Gniadecki R, Iversen L, Bryld L, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol 2015; 29: 1128–1134.
- Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PLoS One 2012; 7: e36342.
- Egeberg A, Iversen L, Gniadecki R, Hvid L, Dam T, Bryld L, et al. Characteristics of patients receiving ustekinumab compared with secukinumab for treatment of moderate-to-severe plaque psoriasis–nationwide results from the DERMBIO registry. J Eur Acad Dermatol Venereol 2017; 31: 1183–1187.
- Loft N, Skov L, Bryld L, Gislason G, Egeberg A. Treatment history of patients receiving biologic therapy for psoriasis – a Danish nationwide study. J Eur Acad Dermatol Venereol 2017; 31: e362–e363.
- Loft ND, Skov L, Rasmussen MK, Gniadecki R, Dam TN, Brandslund I, et al. Genetic polymorphisms associated with psoriasis and development of psoriatic arthritis in patients with psoriasis. PloS One 2018; 13: e0192010.
- Loft N, Skov L, Iversen L, Gniadecki R, Dam T, Brandslund I, et al. Associations between functional polymorphisms and response to biological treatment in Danish patients with psoriasis. Pharmacogenomics J 2018; 18: 494.
- Gniadecki R, Bang B, Bryld L, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–252.
- Egeberg A, Ottosen M, Gniadecki R, Broesby-Olsen S, Dam T, Bryld L, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol 2018; 178: 509–519.
- Hesselvig JH, Egeberg A, Loft ND, Zachariae C, Kofoed K, Skov L. Correlation between dermatology life quality index and psoriasis area and severity index in patients with psoriasis treated with ustekinumab. Acta Derm Venereol 2018; 98: 335–339.
- Loft ND, Egeberg A, Rasmussen MK, Bryld LE, Gniadecki R, Dam TN, et al. Patient-reported outcomes during treatment in patients with moderate-to-severe psoriasis: a danish nationwide study. Acta Derm Venereol 2019; 99: 1224–1230.
- Gottlieb AB, Lacour JP, Korman N, Wilhelm S, Dutronc Y, Schacht A, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two Phase III randomized studies. J Eur Acad Dermatol Venereol 2017; 31: 679–685.
- Chiricozzi A, Balato A, Conrad C, Conti A, Dapavo P, Ferreira P, et al. Secukinumab demonstrates improvements in absolute and relative psoriasis area severity indices in moderate-to-severe plaque psoriasis: results from a European, multicentric, retrospective, real-world study. J Dermatol Treat Treatment 2020; 31: 476–483.
- Mahil S, Wilson N, Dand N, Reynolds N, Griffiths C, Emsley R, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol 2020; 182: 1158–1166.
- Loft N, Egeberg A, Rasmussen MK, Bryld LE, Nissen CV, Dam TN, et al. Response to biologics during the first six months of therapy in biologic-naïve patients with psoriasis predicts risk of disease flares: a Danish nationwide study. Acta Derm Venereol 2021; 101: adv00357.
- Puig L, Dossenbach M, Berggren L, Ljungberg A, Zachariae C. Absolute and relative Psoriasis Area and Severity Indices (PASI) for comparison of the efficacy of ixekizumab to etanercept and placebo in patients with moderate-to-severe plaque psoriasis: an integrated analysis of UNCOVER-2 and UNCOVER-3 outcomes. Acta Derm Venereol 2019; 99: 971–977.
- Mason KJ, Barker JN, Smith CH, Hampton PJ, Lunt M, McElhone K, et al. Comparison of drug discontinuation, effectiveness, and safety between clinical trial eligible and ineligible patients in BADBIR. JAMA Dermatol 2018; 154: 581–588.