SHORT COMMUNICATION
Matching-adjusted Indirect Comparison of Dermatology Life Quality Index 0/1 Response in Trials of Calcipotriol Plus Betamethasone Dipropionate Foam and Cream Formulations in Patients with Psoriasis
Ahmad JALILI1, Henrik THONING2, Marie Y. JABLONSKI BERNASCONI2 and Kim A. PAPP3,4*
1Dermatology & Skin Care Clinic, Buochs, Switzerland, 2LEO Pharma A/S, Ballerup, Denmark, 3Probity Medical Research, Waterloo and 4University of Toronto, Toronto, Ontario, Canada. E-mail: kapapp@probitymedical.com
Citation: Acta Derm Venereol 2024; 104: adv12623. DOI https://doi.org/10.2340/actadv.v104.12623.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: May 10, 2023; Accepted: Dec 4, 2023; Published: Feb 8, 2024
Competing interests and funding: AJ has been a consultant and advisor and/or received speaking fees and/or grants and/or served as an investigator in clinical trials for the following companies: AbbVie, Almirall, Amgen, Boehringer Ingelheim, BMS, Celgene, Eli Lilly, Galderma, GSK, LEO Pharma, Janssen, MSD, Novartis, Sanofi and UCB. HT and MYJB are employees at LEO Pharma. KAP has received honoraria for advisory board, speaker, consultant services, scientific officer and/or steering committees from AbbVie, Akros, Amgen, Bausch Health/Valeant, Boehringer Ingelheim, Celgene, Celltrion, Coherus, Eli Lilly, Forbion, Galderma, Janssen, Kyowa Hakko Kirin, LEO Pharma, Meiji Seika Pharma, Merck (MSD), Merck-Serono, Mitsubishi Pharma, Novartis, Pfizer, Reistone, Sanofi-Aventis/Genzyme, Sandoz, Takeda, UCB, vTv Therapeutics and Xencor; and has received research grants from AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Bristol-Myers Squibb, Can-Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dow Pharma, Eli Lilly, Evelo, Galapagos, Galderma, Genentech, Gilead, GSK, Incyte, Janssen, Kyowa Hakko Kirin, LEO Pharma, Medimmune, Merck (MSD), Merck- Serono, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, Takeda and UCB.
INTRODUCTION
Psoriasis is a common, chronic immune-mediated inflammatory skin disease that negatively impacts patients’ quality of life (QoL). Multiple formulations of once-daily, fixed-combination calcipotriol 50 μg/g (Cal) plus betamethasone dipropionate 0.5 mg/g (BD) are approved first-line psoriasis treatments (1). These Cal/BD treatments include, in order of their appearance on the market, ointment and gel (Dovobet®/Daivobet®/Taclonex®, LEO Pharma A/S, Ballerup, Denmark), aerosol foam (Enstilar®, LEO Pharma A/S, Ballerup, Denmark), generic ointment and gel, and a water-based cream that is restricted to topical treatment of mild-to-moderate psoriasis (Wynzora®, MC2 Therapeutics, Ltd., Guildford, UK). Head-to-head comparisons of Cal/BD foam and Cal/BD gel or ointment showed that the foam was statistically significantly more effective than gel or ointment, according to the Physician’s Global Assessment of disease severity scale (PGA), and modified Psoriasis Area and Severity Index (mPASI) (2, 3). In related head-to-head comparisons, Cal/BD cream was also associated with greater improvement in QoL outcomes vs gel (4, 5). However, there are no head-to-head comparisons of efficacy or patient-reported outcomes (PROs) with Cal/BD foam and Cal/BD cream. Therefore, we conducted matching-adjusted indirect comparisons (MAICs) to compare individual patient data (IPD) available for multiple Cal/BD foam trials and the aggregate data from the EU and US Cal/BD cream trials of adult patients with mild-to-moderate plaque psoriasis. In our previous MAIC analyses focusing on efficacy outcomes, Cal/BD foam showed significantly greater improvements in PGA and mPASI than Cal/BD cream (6). In this follow-up study, we conducted MAICs focusing on patients’ QoL assessed by a score of 0 or 1 in the Dermatology Life Quality Index (DLQI 0/1) in 4 Cal/BD foam trials and the 2 combined Cal/BD cream trials.
MATERIALS AND METHODS (see APPENDIX S1)
RESULTS
After matching to the pooled estimates of patient baseline characteristics from the European Union (EU) and US Cal/BD cream trials (Table SI), the effective sample sizes of the Cal/BD foam trials ranged from 71.6% to 96.4% of the original sample sizes, and most patients from the Cal/BD foam trials (>80%) had “moderate disease” by Investigator’s Global Assessment (IGA) (Tables SII–SV). The weighted baseline characteristics of PSO-ABLE were identical when matched with the respective comparators in the pooled Cal/BD cream trials (i.e. matching of Cal/BD foam vs cream arms, and of anchor Cal/BD gel arms) (Table SII). For the unanchored analyses, the weighted baseline characteristics were either identical (LEO90100-07 and LP0053-1001 randomized controlled trials (RCTs)) or largely similar (PSO-LONG open-label phase and pooled Cal/BD foam trial IPD) to the pooled Cal/BD cream treatment arms (Tables SIII–SV).
In the anchored MAIC of Cal/BD foam vs cream, the percentage of patients with DLQI 0/1 response was greater for both treatments than the comparator Cal/BD gel (Fig. 1A). Treatment with Cal/BD cream for 8 weeks was associated with numerically greater DLQI 0/1 response than with Cal/BD foam for 4 weeks; however, the confidence intervals were wide, and the difference between the 2 treatments was statistically insignificant (Fig. 1A).
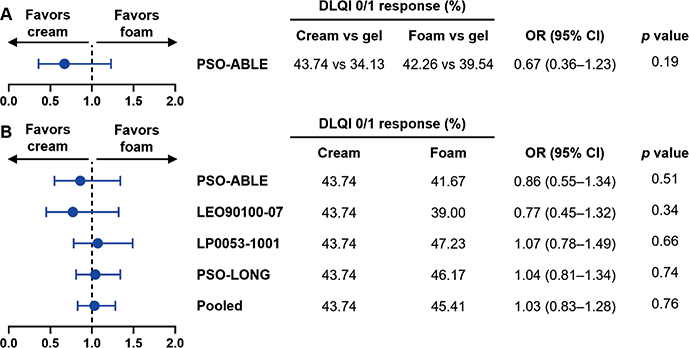
Fig. 1. Forest plots of (A) the anchored matching-adjusted indirect comparison (MAIC) of Dermatology Life Quality Index (DLQI) 0/1 response for Cal/BD foam at Week 4 vs calcipotriol (Cal)/ betamethasone dipropionate (BD) cream at Week 8 with Cal/BD gel as a common comparator and (B) unanchored MAICs of DLQI 0/1 response for Cal/BD foam at Week 4 vs Cal/BD cream at Week 8. (A) Odds ratios presented for achieving DLQI 0/1 response in an anchored MAIC of PSO-ABLE randomized controlled trial (RCT) vs US and EU RCTs of Cal/BD cream. DLQI 0/1 response rates are for the comparators vs Cal/BD gel. (B) Odds ratios (ORs) presented for achieving DLQI 0/1 response in unanchored MAICs of the Cal/BD foam RCTs (PSO-ABLE, LEO90100-07, and LP0053-1001); the PSO-LONG open-label phase; and the pooled Cal/BD foam individual patient data (IPD), vs US and EU RCTs of Cal/BD cream. 95% CI: 95% confidence interval; Pooled: pooled Cal/BD foam treatment groups (PSO-ABLE RCT arm, LEO90100-07 RCT arm, LP0053-1001 RCT arm, PSO-LONG open-label phase).
In the unanchored MAICs with the Cal/BD foam RCT arms (PSO-ABLE, LEO90100-07, and LP0053-1001) vs the Cal/BD cream trials, Cal/BD foam was associated with greater DLQI 0/1 response vs Cal/BD cream in only LP0053-1001 (Fig. 1B). The PSO-LONG open-label phase IPD had the largest effective sample size after matching of all the Cal/BD foam studies (Neff = 548.48, Table SIV) given that randomization of patients to the foam and placebo arms had not yet occurred. The corresponding unanchored MAIC vs Cal/BD cream numerically favoured Cal/BD foam with a narrower confidence interval than for the MAICs discussed above, with similar results observed in the unanchored MAIC of the pooled Cal/BD foam treatment groups vs Cal/BD cream trials (Fig. 1B). Nevertheless, across the 5 unanchored MAICs (of which 3 numerically favoured Cal/BD foam), the difference in DLQI 0/1 response between the 2 treatments was insignificant (Fig. 1B).
DISCUSSION
Our previous MAIC analyses showed that Cal/BD foam was significantly more efficacious than Cal/BD cream according to the PGA and mPASI outcomes (6). In this follow-up study, MAICs of DLQI 0/1 response data of Cal/BD foam and Cal/BD cream trials do not show a significant difference between 4 weeks of Cal/BD foam and 8 weeks of Cal/BD cream, suggesting that both on-label treatments have a similar impact on QoL. Together, our MAICs focusing on efficacy and QoL suggest that Cal/BD foam exhibits superior efficacy and comparable DLQI 0/1 response to Cal/BD cream, but in half the time of treatment.
In contrast, recent MAIC analyses of Cal/BD cream vs foam, concluded that there is a numerical, but insignificant, “trend” for greater DLQI 0/1 response with Cal/BD cream; that both treatments are numerically on par for efficacy (using PGA and PASI-75); and that Cal/BD cream is significantly to almost-significantly better than foam in treatment satisfaction using PRO measures from the Psoriasis Treatment Convenience Scale (PTCS) and the Topical Product Usability Questionnaire (TPUQ) (7). Methodological differences between the analyses focusing on Cal/BD foam and those on Cal/BD cream may contribute to the different conclusions regarding the efficacy and PROs of the 2 treatments.
The indirect comparisons of 4 weeks of Cal/BD foam vs 8 weeks Cal/BD cream reported here are strengthened by the larger collection of Cal/BD foam studies used with DLQI 0/1 response data: 4 separate clinical trials and a pooled analysis with the Cal/BD foam treatment groups, which is greater than the clinical data package available for the recently launched Cal/BD cream. Furthermore, pooling of the 4 trials and controlling for prognostic factors, such as disease severity enhances the MAIC vs Cal/BD cream, as opposed to using only 1 Cal/BD foam trial without said adjustment. In this study, data of severe patients were excluded to compare DLQI 0/1 response vs Cal/BD cream more appropriately since the EU and US Cal/BD cream trials did not have severe patients. In the previous MAICs covering efficacy and PROs (7), only 2 Cal/BD foam studies with DLQI data were used: PSO-ABLE (2, 8), and PSO-INSIGHTFUL, which also assessed treatment satisfaction measured by the TPUQ (9). These indirect comparisons were conducted without the exclusion of severe patients, nor adjustment on prognostic factors such as baseline severity or the other variables listed in Tables SI–SV, as that was not possible with the Bucher’s adjusted indirect comparison method employed (7). Such an adjustment is feasible with the approach detailed here (10) and in a related MAIC of Cal/BD cream vs foam that corroborates the similar impact on QoL of the 2 approved treatments (11). Moreover, Reich et al. included MAICs of DLQI 0/1 response between on- and off-label Cal/BD cream and Cal/BD foam treatments to compare the same treatment durations; the results at 4 (off-label Cal/BD cream) and 8 (off-label Cal/BD foam) weeks numerically favoured Cal/BD cream, but the differences were insignificant (7).
Another explanation for the different conclusions is the ambiguity of the treatment satisfaction comparison. The MAIC focusing on Cal/BD cream matched the 5 PTCS questions with relevant TPUQ questions; however, the TPUQ is comprised of 26 items that evaluate patients’ preference for topical treatment and using a subset may affect the results of the comparison with Cal/BD foam (9). Moreover, the treatment satisfaction questions posed to subjects in the Cal/BD foam and cream trials, though similar, are not identical; thereby making a comparison challenging (7). For instance, the TPUQ was only published with the PSO-INSIGHTFUL trial results (9) and has not been validated further like PTCS used in the Cal/BD cream trials (12).
In conclusion, there are substantial data showing that while Cal/BD foam and Cal/BD cream may be comparable in terms of DLQI performance, Cal/BD foam may achieve these outcomes in half the time. Therefore, nuanced comparisons of treatment satisfaction are necessary to better differentiate between the 2 formulations.
ACKNOWLEDGEMENTS
Medical writing support, including assisting authors with the development of the manuscript drafts and incorporation of comments, was provided by Juliel Espinosa, PhD of Alphabet Health (New York, NY), supported by LEO Pharma A/S, according to Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022). The authors received no honoraria related to the development of this publication.
REFERENCES
- Rudnicka L, Olszewska M, Goldust M, Waśkiel-Burnat A, Warszawik-Hendzel O, Dorożyński P, et al. Efficacy and safety of different formulations of calcipotriol/betamethasone dipropionate in psoriasis: gel, foam, and ointment. J Clin Med 2021; 10: 5589.
- Paul C, Stein Gold L, Cambazard F, Kalb RE, Lowson D, Bang B, Griffiths CEM. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol 2017; 31: 119–126.
- Koo J, Tyring S, Werschler WP, Bruce S, Olesen M, Villumsen J, Bagel J. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris – a randomized phase II study. J Dermatol Treat 2016; 27: 120–127.
- Pinter A, Green LJ, Selmer J, Praestegaard M, Gold LS, Augustin M. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J Eur Acad Dermatol Venereol 2022; 36: 228–236.
- tein Gold L, Green LJ, Dhawan S, Vestbjerg B, Praestegaard M, Selmer J. A phase 3, randomized trial demonstrating the improved efficacy and patient acceptability of fixed dose calcipotriene and betamethasone dipropionate cream. J Drugs Dermatol 2021; 20: 420–425.
- app KA, Thoning H, Gerdes S, Megna M, Brandi H, Jablonski Bernasconi MY, Yélamos O. Matching-adjusted indirect comparison of efficacy outcomes in trials of calcipotriol plus betamethasone dipropionate foam and cream formulations for the treatment of plaque psoriasis. J Dermatolog Treat 2022; 33: 3005–3013.
- eich A, Selmer J, Galván J, Trebbien P, Pi-Blanque A, Danø A, et al. Efficacy, quality of life, and treatment satisfaction: an indirect comparison of calcipotriol/betamethasone dipropionate cream versus foam for treatment of psoriasis. Curr Med Res Opin 2022; 38: 1521–1529.
- Griffiths CE, Stein Gold L, Cambazard F, Kalb RE, Lowson D, Møller A, Paul C. Greater improvement in quality of life outcomes in patients using fixed-combination calcipotriol plus betamethasone dipropionate aerosol foam versus gel: results from the PSO-ABLE study. Eur J Dermatol 2018; 28: 356–363.
- Hong CH, Papp KA, Lophaven KW, Skallerup P, Philipp S. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol 2017; 31: 1876–1883.
- Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health 2012; 15: 940–947.
- Bewley A, Barker E, Baker H, Green W, Avey B, Pi-Blanque A, et al. An anchored matching-adjusted indirect comparison of fixed-dose combination calcipotriol and betamethasone dipropionate (Cal/BDP) cream versus Cal/BDP foam for the treatment of psoriasis. J Dermatol Treat 2022; 33: 3191–3198.
- Feldman SR, Præstegaard M, Andreasen AH, Selmer J, Holm-Larsen T. Validation of the self-reported Psoriasis Treatment Convenience Scale (PTCS). Dermatol Ther (Heidelb) 2021; 11: 2077–2088.