Bullous pemphigoid (BP), an autoimmune blistering skin disease characterized by pruritus, urticarial plaques, blisters, and erosions, is induced by IgG-autoantibodies against the hemidesmosomal proteins BP180 and/or BP230 (1, 2). Erythrodermic BP is an unusual variant with generalized erythroderma (3). Pityriasis rubra pilaris (PRP) is a rare chronic inflammatory papulosquamous disorder of unknown ethology.
CASE REPORT
A 71-year-old Caucasian woman presented with a 6-week history of pruritic erythroderma that started on her left chest. She did not report recent infections, fever, night sweats, or weight loss. Her past medical history included breast cancer, chronic hypersensitivity pneumonitis (CHP) and diabetes. Physical examination showed generalized erythroderma and fine scaling with concomitant blisters around her mastectomy scar (Fig. 1a). Blood tests were unremarkable. A lesional biopsy showed a subepidermal blister and an inflammatory infiltrate containing lymphocytes and eosinophils. Direct immunofluorescence (IF) of a perilesional biopsy revealed linear deposition of C3 and IgG along the basement membrane (Fig. 2a). IgG-autoantibodies bound to the roof of an artificial blister on human salt-split skin (Fig. 2b). Whereas no serum IgG against BP180 NC16A and BP230 was detected by enzyme-linked immunoassay (ELISA), IgG reactivity was observed against the 120-kDa cell-derived soluble ectodomain of BP180 (LAD-1) by immunoblotting (Fig. 2c). An initial diagnosis of erythrodermic BP was made. Treatment included oral antihistamines and topical clobetasol propionate for 2 weeks; however, the skin lesions did not improve. The patient subsequently also developed palmoplantar hyperkeratosis, scaling of the scalp, thickened nails, islands of normal-appearing skin, cervical lymphadenopathy and oedema of the lower legs (Fig. 1b and c), and a biopsy taken from lichenified erythema revealed acanthosis, parakeratosis, papillomatosis, a prominent granular layer, and a dermal perivascular lymphocytic inflammation. These findings were consistent with a diagnosis of PRP. To exclude a relapse of her breast cancer a positron emission tomography-computed tomography (PET-CT) scan was performed, which was unremarkable. Treatment was expanded to include oral acitretin 10 mg per day. However, there was an unsatisfactory improvement. Due to its best effect/side-effect profile, we administered intravenous immunoglobulin (IVIg) 2 g/kg body weight (160 g) over 4 days every 4 weeks. Within the next 2 months scaling, palmoplantar hyperkeratosis and erythroderma improved significantly and no new blisters appeared (Fig. 1d). Exacerbation of her pre-existing CHP required short-term non-invasive ventilation and administration of oral methylprednisolone 1 mg/kg body weight, which was gradually tapered to a maintenance dose of 4 mg. Subsequently, the tyrosine kinase inhibitor nintedanib was started for her interstitial lung disease.
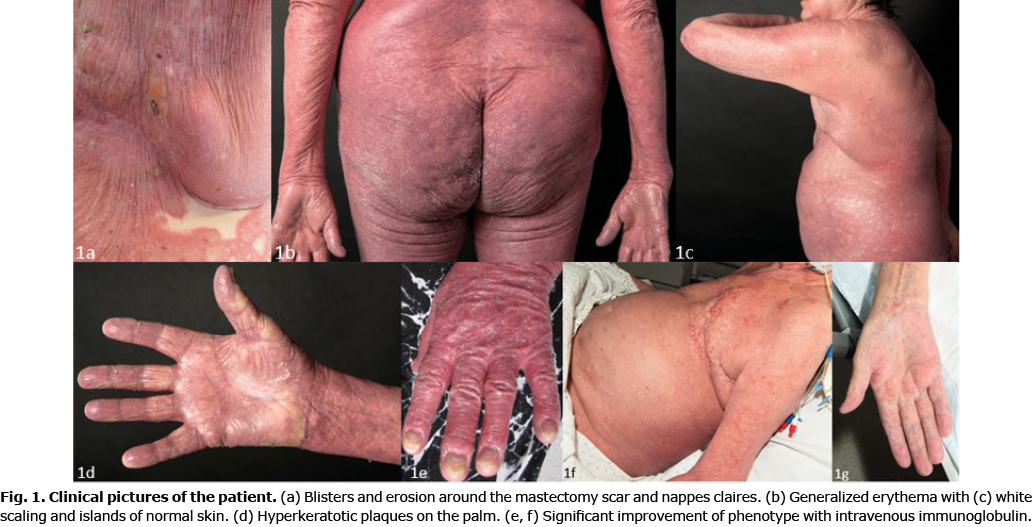
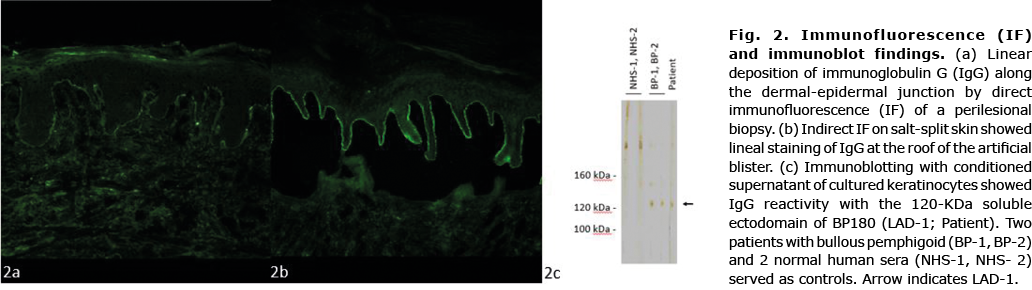
In summary, the patient presented with coinciding PRP and BP with autoantibodies against LAD-1. After initiation of high-dose IVIg, both diseases showed a significant improvement.
DISCUSSION
The coincidence of PRP and BP is very rare; only one patient has previously been reported in the literature (4). BP can rarely present as erythroderma. However, palmoplantar keratoderma and islands of uninvolved skin and the histology were consistent with concurrent PRP. In contrast to a previous report of coinciding BP and PRP (4) with antibodies against BP180 NC16A and BP230, the current patient presented IgG-antibodies against the BP180 ectodomain (LAD-1) outside the immunodominant NC16A domain. BP with antibodies to LAD-1 is very rare; currently only 4 patients have been reported in the literature (5).
Triggers relevant for both BP and PRP remain unknown in the current patient. As both disorders may be associated with malignancies and autoimmune diseases (6), a relapse of her breast cancer or other malignant disease was excluded; a thorough medication history did not identify possible drug triggers. It is conceivable that the erythroderma exposed autoantigens in the epidermis of the current patient, since additional cases with psoriatic erythroderma and LAD-1 BP have been reported (7, 8), and PRP is known to be associated with other autoimmune disorders, including myasthenia gravis, thyroiditis, coeliac disease, and vitiligo.
The use of IVIg is known as an effective treatment of severe forms of autoimmune blistering diseases, dermatomyositis, systemic vasculitic syndromes, lupus erythematodes, toxic epidermal necrolysis or scleromyxoedema (9). It is well tolerated, but can sometimes lead to minor side-effects, such as headache, myalgia, or nausea, which are usually self-limiting (10). Because of a reported higher risk of recurrence or incidence of neoplasia, we decided against TNFα inhibitors as a treatment option (11). Although there is no increased risk for malignancy with ustekinumab, the patient declined this therapy (12).
In conclusion, clinicians should consider the coincidence of BP and PRP in patients with concurrent erythroderma and blistering skin disease. The use of high-dose IVIg is a safe and effective therapeutic option for both diseases.
ACKNOWLEDGEMENTS
We thank the patient for granting permission to publish this observation. We are grateful to Marina Kongsbak-Reim, Lübeck, for providing immunofluorescence images and Randolph Bodenstein, Lübeck, for carrying out the immunoblot.
The authors have no conflicts of interest to declare.
REFERENCES
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet 2013; 381: 320–332.
- Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosae: clinical features, diagnosis, and management. Clin Rev Allergy Immunol 2018; 54: 26–51.
- Chaudhary R, Chaudhary N. A 45-year-old woman with erythroderma and bullous eruption: erythrodermic bullous pemphigoid. J Pharm Biomed Sci 2018; 8: 94–97.
- Genovese G, Muratori S, Berti E, Marzano AV. Bullous erythroderma: novel association of pityriasis rubra pilaris with bullous pemphigoid. Clin Exp Dermatol 2019; 44: 73–75.
- Miyashita K, Iioka H, Miyagawa F, Ishii N, Hashimoto T, Asada H. A case of bullous pemphigoid with IgG antibodies against LAD-1, but not BP180 NC16a domain or BP230. Eur J Dermatol 2018: 28: 248–250.
- Schulze F, Neumann K, Recke A, Zillikens D, Linder R, Schmidt E. Malignancies in pemphigus and pemphigoid diseases. J Invest Dermatol 2015; 135: 1445–1447.
- Goto-Hamano H, Ito K, Sakamoto-Kumura K, Terui T, Ohyama B, Hashimoto T, Hara H. Autoantibodies against multiple epitopes in Bp180 and laminin gamma-1 in subepidermal blistering skin disease associated with psoriatic erythroderma. Indian J Dermatol 2015; 60: 521.
- Roeder C, Driesch PV. Psoriatic erythroderma and bullous pemphigoid treated successfully with acitretin and azathioprine. Eur J Dermatol 1999; 9: 537–539.
- Kerr AC, Ferguson J. Type II adult-onset pityriasis rubra pilaris successfully treated with intravenous immunoglobulin. Br J Dermatol 2007; 156: 1055–1056.
- Hoffmann JHO, Enk AH. High-Dose Intravenous Immunoglobulin in skin autoimmune disease. Front Immunol 2019; 10: 1090.
- Fiorentino D, Ho V, Lebwohl MG, Leite L, Hopkins L, Galindo C, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol 2017; 77: 845–854.e5.
- Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol 2013; 168: 844–854.