ORIGINAL REPORT
Expression Analysis of Retinal G Protein-coupled Receptor and its Correlation with Regulation of the Balance between Proliferation and Aberrant Differentiation in Cutaneous Squamous Cell Carcinoma
Jianglong FENG1–3# , Wei ZHANG1#
, Wei ZHANG1# , Wen ZENG1#
, Wen ZENG1# , Xian DONG1
, Xian DONG1 , Yu WANG1
, Yu WANG1 , Yangguang GU1
, Yangguang GU1 , Yinghua LAN1
, Yinghua LAN1 , Wenxiu YANG3 and Hongguang LU1
, Wenxiu YANG3 and Hongguang LU1
1Department of Dermatology, Affiliated Hospital of Guizhou Medical University, 2School of Public Health, Guizhou Medical University, 3Department of Pathology, Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
#These authors contributed equally to this work.
Retinal G protein-coupled receptor (RGR), a photosensitive protein, functions as a retinal photoisomerase under light conditions in humans. Cutaneous squamous cell carcinoma (cSCC) is linked to chronic ultraviolet exposure, which suggests that the photoreceptor RGR may be associated with tumorigenesis and progression of squamous cell carcinoma (SCC). However, the expression and function of RGR remain uncharacterized in SCC. This study analysed RGR expression in normal skin and in lesions of actinic keratosis, Bowen’s disease and invasive SCC of the skin with respect to SCC initiation and development. A total of 237 samples (normal skin (n = 28), actinic keratosis (n = 42), Bowen’s (n = 35) and invasive SCC (n = 132) lesions) were examined using immunohistochemistry. Invasive SCC samples had higher expression of RGR protein than the other samples. A high immunohistochemical score for RGR was associated with increased tumour size, tumour depth, Clark level, factor classification, and degree of differentiation and a more aggressive histological subtype. In addition, RGR expression was inversely correlated with involucrin expression and positively correlated with proliferating cell nuclear antigen (PCNA) and Ki67 expression. Furthermore, RGR regulates SCC cell differentiation through the PI3K-Akt signalling pathway, as determined using molecular biology approaches in vitro, suggesting that high expression of RGR is associated with aberrant proliferation and differentiation in SCC.
SIGNIFICANCE
Retinal G protein-coupled receptor is a photosensitive protein that senses light and plays a role in retinal metabolism in humans. Exposure to sunlight is the strongest risk factor for cutaneous squamous cell carcinoma. This study showed that high expression of retinal G protein-coupled receptor is significantly associated with aggressive biological behaviours in cutaneous squamous cell carcinoma through regulation of the balance between proliferation and aberrant differentiation. These findings provide novel insight into the role of the photosensitive protein retinal G protein-coupled receptor in skin tumours, revealing its important role in promoting cutaneous squamous cell carcinoma initiation and development.
Citation: Acta Derm Venereol 2024; 104: adv13213. DOI https://doi.org/10.2340/actadv.v104.13213.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: May 12, 2023; Accepted: Oct 3, 2023; Published: Jan 26, 2024
Corr: Hongguang Lu, Department of Dermatology, Affiliated Hospital of Guizhou Medical University, No. 28 Guiyi Road, Guiyang, Guizhou, 550001, PR China. E-mail: hongguanglu@hotmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Opsins, a large family of cell surface photoreceptors that sense distinct wavelengths of light to drive signalling cascades with retinal as a ligand, play multiple roles in phototransduction in the visual process (1). The opsin family can be divided into the visual and non-visual opsin subfamilies. Retinal G protein-coupled receptor (RGR), expressed in Müller glia and the retinal pigment epithelium (RPE), belongs to the non-visual opsin subfamily (2). In cooperation with retinol dehydrogenase-10, RGR in the RPE and Müller cells can photoisomerize all-trans-retinaldehyde to 11-cis-retinaldehyde under daylight conditions to continuously supply the visual chromophore retinal to retinal photoreceptors, enabling the eye to retain responsiveness to daylight (3–5). Collectively, previous findings have demonstrated that RGR plays an important role in the regeneration of visual chromophores under sustained light conditions. However, in extraocular tissues, RGR remains one of the few members of the opsin family with an unknown function. Our recent study, using immunohistochemical (IHC) staining, found that RGR is more highly expressed in proliferative diseases, including psoriasis, seborrhoeic keratosis and cutaneous squamous cell carcinoma (cSCC), in a small number of samples compared with normal skin (NS) tissues (6). Moreover, our previous study revealed that RGR expression in ultraviolet (UV)-exposed skin sites is higher than that in non-exposed skin sites. Furthermore, RGR knockdown in epidermal keratinocytes led to the inhibition of cell proliferation and migration, increasing cellular apoptosis in vitro (6). These findings indicate that RGR might be a marker related to cell proliferation in keratinocytes. However, the expression characteristics and function of RGR in extraocular tissues, such as skin and skin appendages, have yet to be determined.
Exposure to sunlight is the strongest risk factor for cSCC, because mutations in genes such as TP53, CDKN2A, and Ras induced by exposure to UV radiation (UVR) play an important role early in the pathogenesis of cSCC (7). TP53 mutation induced by ultraviolet B (UVB) exposure has been confirmed to result in the formation of cyclobutane pyrimidine dimers (CPDs) by the direct absorption of UVB by DNA (8, 9). Both visible light and UVA radiation induce CPD formation via oxidative stress and reactive nitrogen species, which do not directly damage DNA (8). However, whether opsin, as the photoreceptor, mediates visible light and/or UVA sensing to promote cSCC tumorigenesis and progression remains unknown.
Our study and other studies demonstrated that specific opsins, including OPN1, OPN2, OPN3, OPN4, OPN5, peropsin and RGR, are expressed in the epidermis and dermis in humans (10–14). and showed that some opsins, such as OPN3 and peropsin, are associated with processes related to cell survival, including proliferation, migration, apoptosis and differentiation, via light-independent or light-dependent signalling (6, 11, 12, 14). Hence, it can be hypothesized that the photoreceptor RGR may be associated with UV-related cSCC. The aim of the current study was to further identify the expression characteristics of RGR and its potential role in cSCC with respect to cSCC initiation and development from precancerous lesions of actinic keratosis (AK) to Bowen’s disease (BD) in situ to invasive SCC. Expression analysis and characterization of RGR was performed in NS tissues and lesions of AK, BD and invasive SCC of the skin and the association of RGR with clinicopathological features was investigated.
MATERIALS AND METHODS
Data collection
The study cohort comprised 237 samples of NS tissues (n = 28) and lesions of AK (n = 42), BD (n = 35) and invasive SCC (n = 132) obtained from the Affiliated Hospital of Guizhou Medical University. Detailed information on the samples is shown in Table SI. Haematoxylin and eosin (H&E)-stained sections were reviewed and evaluated, and samples meeting the criteria for the appropriate diagnoses of AK, BD and SCC were selected for study. Archived formalin-fixed, paraffin-embedded (FFPE) blocks were sliced into 4-µm sections for IHC staining, an RNAscope assay and multiplex immunofluorescence staining. Details of the methods of IHC staining and further semiquantitative assessment, RNAscope RGR mRNA in situ hybridization assay, and multiplex immunofluorescence staining can be found in previous reports (15–18). Detailed materials and methods are also provided in Appendix S1. The study was approved by the ethics committees of Affiliated Hospital of Guizhou Medical University (approval number: 2021541).
Cell culture
The A431 cell line (human cSCC cells) was purchased from ATCC and maintained in Dulbecco’s Modified Eagle Medium (Cat. No. 0030034DJ; Gibco, Grand Island, USA) supplemented with 10% fetal bovine serum (FBS, Cat.No. FBSST-01033-500, Oricell, Guangzhou, China), 100 U/mL penicillin, and 100 µg/mL streptomycin (Cat. 3810-74-0 Sigma-Aldrich; Merck KGaA, Shanghai, China) in a 37°C humidified incubator containing 5% CO2.
Lentiviral infection
According to the standard lentiviral production protocol (GeneChem, Shanghai, China), lentiviral particles (LV-RGR-RNAi [103556-1] and LV-control-RNAi [CON313]) were produced via transfection of GV493 vectors into HEK293T cells. Lentiviral particles (LV-RGR [KL29757-4] and LV-control [CON335]) were also produced via transfection of GV358 vectors into HEK293T cells. The viral supernatant was harvested for the subsequent studies. Cells were cultured in 12-well plates at a density of 3 × 105 cells/well at 37°C in 5% CO2 for 24 h. Lentiviral particles (multiplicity of infection = 10 pfu/mL) were added to the medium to infect A431 cells at 30–40% confluence in 12-well plates. After 72 h, the medium was replaced with fresh medium for continued culture. When 80% of the cells showed green fluorescence, selection pressure medium containing 5 µg/ml puromycin was added. Within 3–4 weeks, puromycin-resistant cell colonies were collected for measurement of the lentiviral transduction efficiency by western blot (WB) and real-time quantitative PCR (RT-qPCR) analyses.
RNA isolation and sequencing
Total RNA was extracted using TriPure (Cat #: 11667165001 Roche,Shanghai, China), and total RNA from the 4 groups of cell samples (LV-RGR-RNAi, LV-control-RNAi, LV-RGR, LV-control) were sequenced on an Illumina platform (NEB, ,San Diego, CA, USA], Catalog #: E7370L). According to the effective library concentration and amount of data needed, quality control and bioinformatic analysis were performed. RT-qPCR and WB analyses were used to validate the differential expression of candidate genes related to cell differentiation. Detailed materials and methods are provided in Appendix S1.
Statistical analysis
GraphPad Prism (GraphPad Software, San Diego, CA, USA version 8.0) software was used for statistical analysis. Continuous variables are presented as means±standard deviations (SDs) or as medians with interquartile ranges (IQRs) when the distribution was skewed. Comparisons between the means of 2 groups or among the means of more than 2 groups were performed by student’s t-tests and 1-way analysis of variance (ANOVA), respectively. Both the Mann–Whitney U test (1 groups) and the Kruskal–Wallis test (more than 2 groups) were used for comparisons of data with a non-parametric distribution. Differences were considered statistically significant when p < 0.05.
RESULTS
Retinal G protein-coupled receptor expression in human normal skin tissue
In skin sections, immunofluorescence staining showed that RGR was expressed throughout the epidermis except in the stratum corneum, with more prominent expression in the basal layer (Fig. 1A). RGR was also expressed in the dermis and skin appendages, in which it was localized primarily in the epithelial cells of sebaceous glands, sweat glands, and hair follicles (Fig. 1B, C). RGR was expressed weakly in vascular endothelial cells, as determined by co-staining with CD31 (a vascular endothelial cell marker), compared with hair follicles (Fig. 1D). Notably, RGR expression in ductal epithelial cells was significantly stronger than that in glandular epithelial cells of eccrine glands labelled with carcinoembryonic antigen (CEA) (Fig. 1B, Fig. S1). These results showed that the RGR protein was differentially expressed in different epithelial cell types of skin appendages or the epidermis.
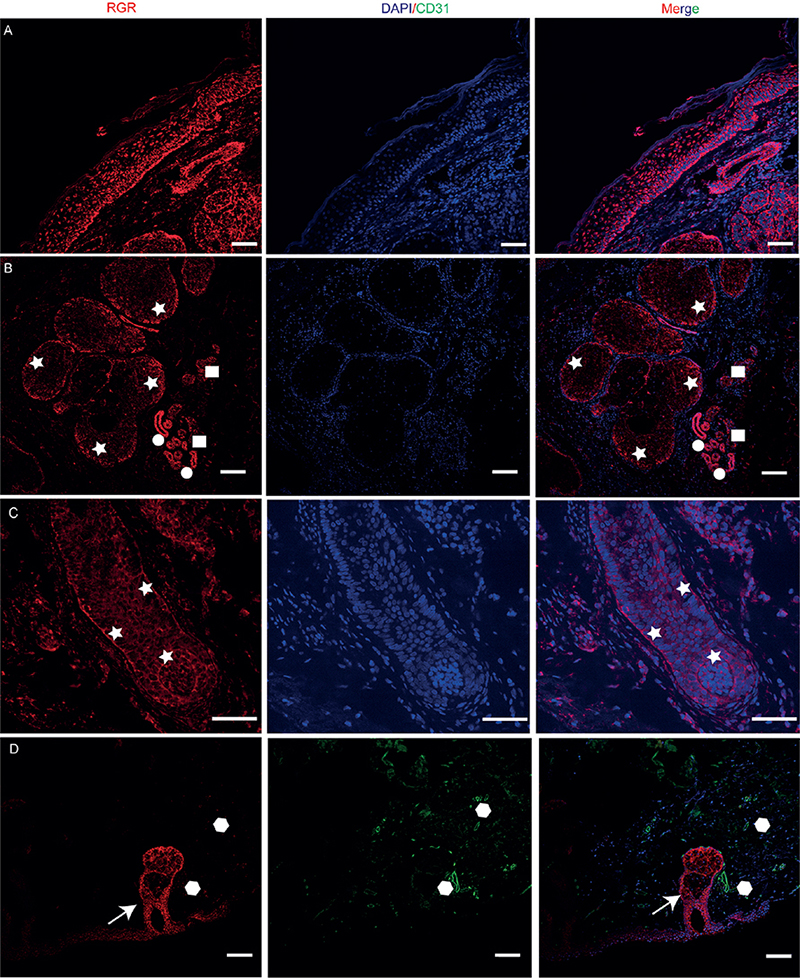
Fig. 1. Immunofluorescence analysis of retinal G protein-coupled receptor (RGR) (red) protein expression in normal skin (NS) and skin appendage tissue. (A) Selected co-staining image showing RGR expression mainly in the basal cell layer of the epidermis. RGR was also expressed in skin appendages, localized primarily in the epithelial cells of sebaceous glands ((B) asterisks), eccrine glands (B) square dots: sweat ducts; round dots: sweat glands), and hair follicles (C) asterisks) (blue, 4’,6-diamidino-2-phenylindole). (D) RGR was expressed weakly in the vascular endothelial cells, as determined by co-staining for CD31 (green, polygonal dots) (arrow, hair follicles). Scale bars: 100 µm.
Clinicopathological data corresponding to actinic keratosis, Bowen’s disease and invasive squamous cell carcinoma samples
The general patient characteristics corresponding to the 237 samples, including NS and AK, BD and invasive SCC lesions, are highlighted in Table SI. The mean ages at diagnosis of the patients with AK, BD and SCC were 74.1, 74.7 and 70.7 years, respectively, with corresponding male:female ratios of 1:1.2, 1:1.9 and 1:0.8. The mean age of the individuals providing the NS samples was 60.1 years, with a male:female ratio of 1:1 (Table SI). The SCC samples were classified into 8 histological subtypes: common (48.5%), desmoplastic (12.9%), acantholytic epithelioid (9.1%), adenosquamous (3.0%), spindle cell (9.8%), clear cell (1.5%), verrucous (3.0%), and keratoacanthoma-like (12.1%) (Fig. S2). In terms of the degree of differentiation (using the classification recommended by the WHO) (19), well-differentiated SCC was the most common subtype, accounting for 48.5% of the invasive SCC samples. For risk stratification of cSCC, tumours with a size of > 20 mm, depth of > 2 mm or Clark level of IV or V were considered high-risk (20). Tumour classification (Brigham and Women’s Hospital tumour classification system) was based on clinical and pathological risk factors (20, 21). All patients were Chinese and only accepted surgical treatment.
Analysis of retinal G protein-coupled receptor expression in normal skin and actinic keratosis, Bowen’s disease and squamous cell carcinoma lesion tissues
RGR expression and localization were first examined in NS and AK, BD and SCC lesion tissues. As shown in Fig. 2, RGR protein expression was observed mainly in a membranous and cytoplasmic pattern in all tissues. RGR expression decreased gradually from the basal layer to the granular layer in NS (Fig. 2). The median [IQR] IHC staining scores for RGR differed significantly between benign NS (55.0 [22.5–90.0]), precancerous AK lesions (60.0 [27.5–82.5]), BD in situ lesions (90.0 [60.0–180.0]), and invasive SCC lesions (100.0 [80.00–160.0]) (p < 0.0001) (Fig. 2). The median IHC score for RGR was the highest in the invasive SCC group and the lowest in the NS group (Table I, Fig. 2).
| Variable | Mean or median RGR staining score | p-values |
| Sex (Mean ± SEM) | p = 0.219 | |
| Male | 106.3 ± 6.66 | |
| Female | 118.5 ± 7.23 | |
| Age (years) (Mean ± SEM) | p = 0.139 | |
| < 65 | 98.33 ± 9.78 | |
| ≥ 65 | 115.7 ± 5.6 | |
| Tumor size (mm) (Mean ± SEM) | p = 0.005** | |
| ≤ 20 | 82.59 ± 8.96 | |
| > 20 | 124.1 ± 5.41 | |
| Tumor depth (mm) (Mean ± SEM) | p = 0.0208* | |
| ≤ 2.00 | 87.14 ± 7.7 | |
| 2.00–6.00 | 122.8 ± 7.49 | |
| > 6.00 | 116.1 ± 11.03 | |
| Clark level (Mean ± SEM) | p = 0.002** | |
| I | 80.29 ± 8.57 | |
| II | 113.30 ± 8.44 | |
| III | 127.1 ± 7.74 | |
| IV | 144.60 ± 18.42 | |
| Degree of differentiatione (Mean ± SEM) | p ≤ 0.005** | |
| Poorly differentiated | 144.70 ± 16.30 | |
| Moderately differentiated | 109.70 ± 8.66 | |
| Well differentiated | 91.67 ± 5.41 | |
| Tumor classificationf (Mean ± SEM) | p = 0.002** | |
| T1 | 68.06 ± 5.05 | |
| T2a | 99.05 ± 9.20 | |
| T2b–T3 | 146.2 ± 10.24 | |
| Histologic subtype (Median [IQR]) | p = 0.001** | |
| Common | 90.0 [60.00, 120.00] | |
| Desmoplastic | 140.00 [80.00, 185.00]a,b | |
| Acantholytic | 110.0 [90.00, 175.00] | |
| Adenosquamous | 255.00 [165.00, 270.00]a,b,c,d | |
| Spindle cell | 140.00 [120.00, 185.00]a,b | |
| Clear cell | 155.00 [100.00, 210.00] | |
| Verrucous | 130.00 [105.00, 155.00] | |
| Keratoacanthoma-like | 80.00 [47.5, 100.00] | |
| aCommon vs desmoplastic group p = 0.049; adenosquamous group p < 0.0001; spindle cell group p = 0.0244. bKeratoacanthoma-like vs desmoplastic group p = 0.0199; adenosquamous group p < 0.0001; vs spindle cell group p = 0.0096. cDesmoplastic vs adenosquamous group p = 0.0228. dAcantholytic vs adenosquamous group p = 0.0189. eHistological differentiation by World Health Organization (WHO) classification. fBrigham and Women’s Hospital tumour classification system: T1: 0 risk factors; T2a: 1 risk factor; T2b: 2–3 risk factors; T3: 4 risk factors or bone invasion. Risk factors included a tumour diameter of 2 cm or more, poorly differentiated histology, perineural invasion, and tumour invasion beyond the subcutaneous fat (excluding bone, which automatically upgrades the classification to T3). SEM: standard error of the mean; IQR: interquartile range. *p < 0.05, **p < 0.01. | ||
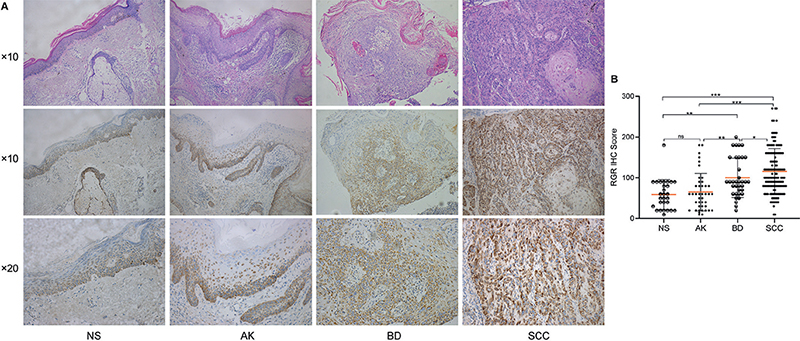
Fig. 2. Immunohistochemical (IHC) analysis of retinal G protein-coupled receptor (RGR) in normal skin (NS) and lesions of actinic keratosis (AK), Bowen’s disease (BD) and squamous cell carcinoma (SCC). (A) IHC staining of RGR in representative samples from NS to AK to BD to SCC. (B) RGR IHC staining score in NS, AK, BD and SCC samples. The RGR IHC score differed significantly among NS, AK, BD and SCC. *p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.001, ns: non-significant (original magnification: HE×10; IHC×10).
Association between retinal G protein-coupled receptor expression and clinical/histopathological variables in squamous cell carcinoma
In addition, associations between the IHC scores for RGR and clinicopathological variables were analysed in patients with invasive SCC (tumour size, Clark level, tumour classification, degree of differentiation, and histological subtype), all of which were found to be significantly correlated with RGR expression (Table I). A statistically significant difference in the IHC score was noted in the comparison between the Clark level I and Clark level IV subgroups (p = 0.002). According to the guidelines of care for the management of cSCC, the patients were divided into 2 groups based on the clinical size of the lesion (< 20 mm and ≥ 20 mm) (20), and the RGR scores were compared (p = 0.005). the association between RGR expression and SCC tumour classification was also estimated based on the Brigham and Women’s Hospital tumour classification system (T0, T1, T2a, T2b, and T3) (20). There was a statistically significant difference between the < T2a and ≥ T2ab groups (p = 0.002). Moreover, RGR protein expression was compared among 8 histological subtypes of SCC (Fig. S3). The mean RGR staining score was highest in the adenosquamous subtype (median [IQR]) (255 [165.0–270.0]) and lowest in the keratoacanthoma-like subtype (median [IQR]) (80.0 [47.50–100.0]). In addition, there were statistically significant differences between histological subtypes (e.g., adenosquamous vs common (p < 0.001); adenosquamous vs keratoacanthoma-like (p < 0.001); adenosquamous vs desmoplastic (p = 0.0228); adenosquamous vs acantholytic epithelioid (p = 0.0189)) of SCC, suggesting that RGR expression differs significantly based on the degrees of proliferation, differentiation and invasion of histological subtypes of SCC. In addition, no associations were found for other histopathological features, including age, sex, exposure of anatomical site and lymphovascular invasion status (Table I). These results suggest that high RGR expression is associated with tumorigenesis and progression in SCC, probably influencing the abnormal proliferation and differentiation of skin keratinocytes.
Retinal G protein-coupled receptor expression correlates with cell proliferation and aberrant differentiation in squamous cell carcinoma
Moreover, a high RGR staining score was positively associated with poorly differentiated SCC compared with well to moderately differentiated SCC (p < 0.005). The mRNA expression level of RGR was also assessed in SCC tissues with different differentiation stages by the RNAscope method. As shown in Fig. S4, the trend in RGR mRNA expression across the differentiation categories of SCC was consistent with the RGR protein expression pattern. In addition, RGR expression in well-differentiated regions of SCC was significantly lower than in poorly differentiated regions in the same sample, as determined using IHC assays (Fig. S4). Similarly, in NS, RGR expression decreased gradually from the basal layer to the stratum corneum (Fig. 3). However, the expression of involucrin, a marker of squamous differentiation, showed a contrasting trend in NS and SCC tissues (Fig. 3), consistent with the results of multiplex immunofluorescence analysis RGR (red) and involucrin (green) in SCC tissues (Fig. S5). RGR expression was inversely correlated with involucrin (differentiation marker) expression (r = –0.356, p = 0.003) by Spearman correlation analysis, while it was positively correlated with proliferating cell nuclear antigen (PCNA) (r = 0.683, p = 0.001) and Ki67 (r = 0.548, p = 0.001) expression (Fig. 4). Together, these results suggest that upregulation of RGR expression is associated with proliferation and aberrant differentiation in SCC, possibly through tipping the balance between these 2 factors.
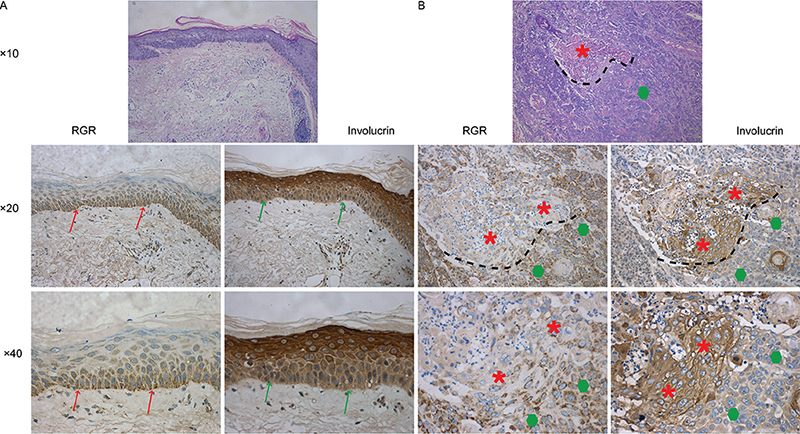
Fig. 3. Immunohistochemical (IHC) analysis of retinal G protein-coupled receptor (RGR) and involucrin in a representative lesion of squamous cell carcinoma (SCC). (A) The expression of RGR was increased in the basal layer (red arrow) compared with the granular layer in normal skin (NS). In contrast, the expression of involucrin was decreased in the basal layer of NS (green arrow). (B) RGR expression in well-differentiated regions (red asterisks) of squamous cell carcinoma (SCC) was significantly lower than that in poorly differentiated regions (green polygons) in the same sample (original magnification: HE×10; IHC, ×20, ×40 ).
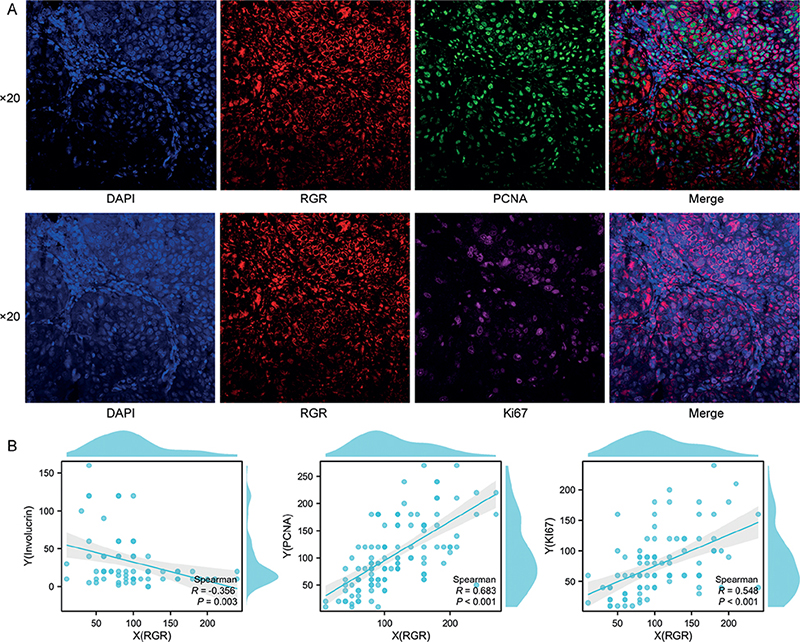
Fig. 4. (A) Immunofluorescence analysis of squamous cell carcinoma (SCC) tissue costained for retinal G protein-coupled receptor (RGR) (red) and proliferating cell nuclear antigen (PCNA) (green) or Ki67 (pink). Selected co-staining image showing RGR expression mainly in the cancer cell membrane (red). (B) Correlation analysis of IHC scores showed that RGR expression was significantly and positively correlated with PCNA and Ki67 expression, but inversely correlated with involucrin expression in squamous cell carcinoma (SCC) tissues (original magnification: ×20).
Effect of retinal G protein-coupled receptor expression on squamous cell carcinoma cell differentiation and the Akt signalling pathway
As shown in Fig. 5, Gene Ontology (GO) biological process (BP) enrichment analysis of the RNA sequencing data showed notable differences in enrichment of the term “cell differentiation” between the vector and RGR3556 (low RGR expression in A431 cells) groups and between the vector and RGR29757 (RGR overexpression in A431 cells) groups. Furthermore, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis revealed that the top 20 differentially enriched KEGG pathways between the vector and RGR3556 groups included the “Formation of the cornified envelope” and “Keratinization” pathways, as shown in the scatterplot. In addition, KEGG enrichment analysis of differentially expressed genes between the vector groups and RGR29757 groups revealed that RGR was closely associated with the PI3K-Akt pathway. Previous studies have shown that the PI3K-Akt signalling pathway is involved in regulating involucrin expression and cell differentiation (22, 23). Based on our bioinformatic analyses of RNA sequencing data and other previous studies, it is reasonable to speculate that RGR might affect involucrin expression to regulate cell differentiation in SCC through the PI3K-Akt signalling pathway. Thus, this study further validated the differential expression of candidate genes related to SCC cell differentiation by RT-qPCR and WB analyses. The results showed that RGR was related to SCC cell differentiation through the PI3K-Akt signalling pathway. The inhibition of RGR was correlated with lower protein levels of p-Akt and Akt, but a higher level of involucrin, and overexpression of RGR exhibited the opposite correlations (Fig. 5C, D). Moreover, IHC staining demonstrated that the protein levels of RGR, phosphorylated Akt, and Akt in poorly differentiated SCC tissue were higher than those in well-differentiated regions of SCC (Fig. 5E–F). These results suggest that RGR regulates SCC cell differentiation via the PI3K-Akt signalling pathway.
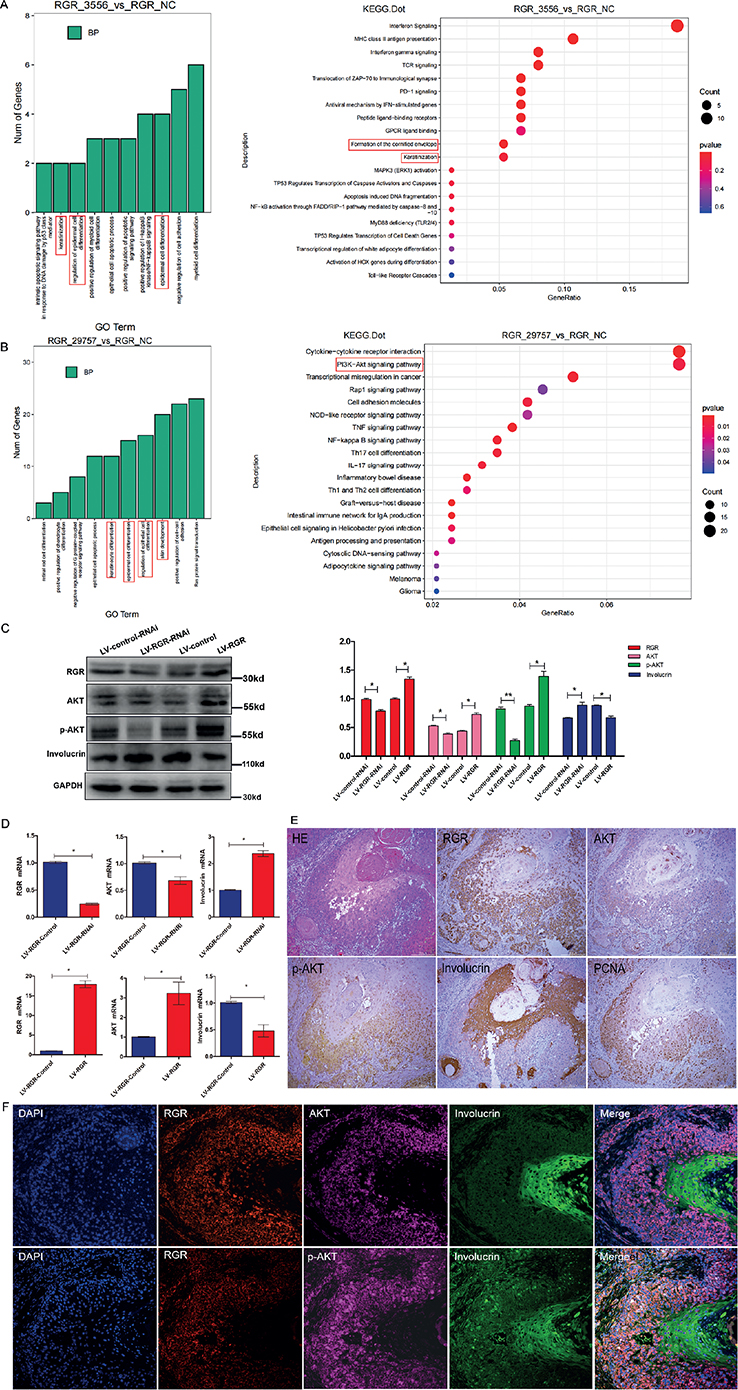
Fig. 5. The effect of retinal G protein-coupled receptor (RGR) expression on squamous cell carcinoma (SCC) cell function and the PI3K-Akt pathway. Transcriptome sequencing and bioinformatic analysis data showing that “cell differentiation” was identified as significantly differentially enriched (A) between the vector and RGR3556 (low RGR expression in A431 cells) groups and (B) between the vector and RGR29757 (RGR overexpression in A431 cells) groups in Gene Ontology (GO) biological process (BP) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, respectively. Following inhibition (LV-RGR-RNAi) and overexpression (LV-RGR) of RGR in A431 cells, (C) western blotting and (D) real-time quantitative PCR (RT-qPCR) were performed to analyse changes in RGR, Akt, p-Akt and involucrin mRNA expression levels; GAPDH was used as a loading control. IHC ((E) original magnification: ×10) and immunofluorescence ((F) original magnification: ×20) analyses showed differences in the levels of RGR, Akt, p-Akt and involucrin between well-differentiated regions of SCC and poorly differentiated regions in the same sample.
DISCUSSION
Cutaneous SCC remains a global public health threat. According to the latest Global Burden of Disease Study, the total number of SCC cases worldwide was approximately 2.4 million in 2019 (24). SCC is characterized by high invasiveness, heterogeneity, and incidence in sun-exposed areas (25); however, the pathogenesis of SCC is incompletely elucidated. RGR, as a photosensitive protein, mediates visual chromophore regeneration by sensing light stimulation in the human eye (26), but the biological function of RGR in extraocular tissues, including tumours, remains unclear. Moreover, the expression and characterization of RGR in various extraocular tissues have rarely been reported. Based on our recent study, RGR expression was first identified in human skin and lesions of some proliferative skin diseases. RGR knockdown induced the inhibition of proliferation and migration, increasing apoptosis in human epidermal keratinocytes (6). Thus, it is reasonable to speculate that the photoreceptor RGR could affect tumorigenesis and progression in light-related skin cancers. Indeed, RGR is highly expressed in SCC tissues compared with paired adjacent NS tissues (6). In addition, our previous study determined that high expression of OPN3, another member of the opsin family, is significantly associated with acral lentiginous melanoma and a metastatic phenotype as well as a poor prognosis (15). De Assis et al. (27) recently demonstrated that OPN4 acts as an oncogene in cutaneous melanoma by impairing cell cycle progression and reducing melanocyte-inducing transcription factor expression.
On the basis of this promising finding, in this study, we first analysed RGR expression in the disease spectrum of aberrant proliferation of keratinocytes from AK to BD to invasive SCC. These results revealed that RGR expression tended to increase across the spectrum from precancerous AK to BD in situ to invasive SCC, suggesting that high expression of RGR may play a role in oncogenesis and/or tumour progression. In particular, the expression of RGR in SCC tissues was markedly associated with some validated risk factors, including tumour size, Clark level, risk classification, degree of differentiation, and histological subtype (20, 28). These results provide novel insight into the function of RGR in skin tumours, demonstrating its supporting role in promoting cSCC initiation and development.
Moreover, to further expand the understanding of the relationship between differentiation and RGR, we assessed the aberrant differentiation of SCC by involucrin staining. Involucrin, a marker of squamous differentiation, is expressed mainly by irreversibly differentiated keratinocytes in the stratum corneum and has been found to be markedly associated with aberrant differentiation in SCC (29, 30). Comparison of RGR expression among different regions with different degrees of differentiation in the same sample showed that the degree of differentiation was inversely correlated with RGR expression, but positively correlated with involucrin expression. Furthermore, the relationship between RGR expression and cell proliferation was evaluated in SCC by co-staining for PCNA and Ki67. Both PCNA and Ki-67 are well known as cell proliferation markers in human tumours (31, 32). PCNA, expressed in the G1 and S phases of the cell cycle, is an essential factor in DNA replication and repair (32). Ki67 is present during all active phases of the cell cycle (G1, S, G2, and mitosis) but not in the quiescent or resting G0 phase (31). By analysing the associations of RGR with proliferation and differentiation markers in SCC and integrating these results with our previous finding that RGR knockdown induced the proliferation inhibition and G1/S arrest in keratinocytes (6), we concluded that RGR may play a role in regulating the balance of SCC cell proliferation and differentiation. Furthermore, the effects of reduced expression or overexpression of RGR on molecular pathways and markers related to SCC cell proliferation and differentiation were examined through in vitro cell and molecular biology methods. Silencing of RGR was correlated with lower levels of p-Akt and Akt, but a higher level of involucrin in the SCC cell line, and overexpression of RGR exhibited the opposite correlations, suggesting that RGR regulates cell differentiation in SCC via the PI3K-Akt signalling pathway. Taken together, these results imply that upregulation of RGR contributes to tumorigenesis and progression in SCC. RGR may act as a tumour-promoting gene in SCC by regulating the balance between cell proliferation and differentiation.
In conclusion, this study demonstrated that RGR expression was upregulated and was associated with clinicopathological characteristics of invasiveness in SCC. The function of RGR is closely associated with cancer cell proliferation and aberrant differentiation. This study provides novel insight into the association of RGR with aberrant differentiation in SCC.
ACKNOWLEDGEMENTS
The study was approved by the ethics committees of our institution (Affiliated Hospital of Guizhou Medical University; Approval number 2021541) and was performed according to the Declaration of Helsinki. All of the participants provided written informed consent.
This study was supported by the National Natural Science Foundation of China (grant numbers: 82060568, 82173397, 82260615) and Natural Science Foundation of Guizhou Province (grant award numbers: ZK2022413, ZK2022420, ZK2022449).
The datasets used and/or analysed in the current study are available from the corresponding author upon reasonable request.
REFERENCES
- Terakita A. The opsins. Genome Biol 2005; 6: 213.
- Pandey S, Blanks JC, Spee C, Jiang M, Fong HK. Cytoplasmic retinal localization of an evolutionary homolog of the visual pigments. Exp Eye Res 1994; 58: 605–613.
- Hao W, Fong HK. The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem 1999; 274: 6085–6090.
- Morshedian A, Kaylor JJ, Ng SY, Tsan A, Frederiksen R, Xu T, et al. Light-driven regeneration of cone visual pigments through a mechanism involving rgr opsin in Müller glial cells. Neuron 2019; 102: 1172–1183.e5.
- Zhang J, Choi EH, Tworak A, Salom D, Leinonen H, Sander CL, et al. Photic generation of 11-retinal in bovine retinal pigment epithelium. J Biol Chem 2019; 294: 19137–19154.
- Gu Y, Wang Y, Lan Y, Feng J, Zeng W, Zhang W, et al. Expression of retinal g protein-coupled receptor, a member of the opsin family, in human skin cells and its mediation of the cellular functions of keratinocytes. Front Cell Dev Biol 2022; 10: 787730.
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018; 78: 237–247.
- Austin E, Geisler AN, Nguyen J, Kohli I, Hamzavi I, Lim HW, et al. Visible light. Part I: Properties and cutaneous effects of visible light. J Am Acad Dermatol 2021; 84: 1219–1231.
- Corchado-Cobos R, García-Sancha N, González-Sarmiento R, Pérez-Losada J, Cañueto J. Cutaneous squamous cell carcinoma: from biology to therapy. Int J Mol Sci 2020; 21: 2956.
- Lan Y, Wang Y, Lu H. Opsin 3 is a key regulator of ultraviolet A-induced photoageing in human dermal fibroblast cells. Br J Dermatol 2020; 182: 1228–1244.
- Wang Y, Lan Y, Lu H. Opsin3 downregulation induces apoptosis of human epidermal melanocytes via mitochondrial pathway. Photochem Photobiol 2020; 96: 83–93.
- Castellano-Pellicena I, Uzunbajakava NE, Mignon C, Raafs B, Botchkarev VA, Thornton MJ. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers Surg Med 2019; 51: 370–382.
- Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochem Photobiol 2015; 91: 117–123.
- Toh PPC, Bigliardi-Qi M, Yap AMY, Sriram G, Stelmashenko O, Bigliardi P. Expression of peropsin in human skin is related to phototransduction of violet light in keratinocytes. Exp Dermatol 2016; 25: 1002–1005.
- Zeng W, Zhang W, Feng J, He X, Lu H. Expression of OPN3 in acral lentiginous melanoma and its associated with clinicohistopathologic features and prognosis. Immun Inflamm Dis 2021; 9: 840–850.
- Fitzgibbons PL, Dillon DA, Alsabeh R, Berman MA, Hayes DF, Hicks DG, et al. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch Pathol Lab Med 2014; 138: 595–601.
- Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012; 14: 22–29.
- Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 2014; 70: 46–58.
- Bonhin RG, Carvalho GMd, Guimarães AC, Chone CT, Crespo AN, Altemani AMdAM, et al. Histologic correlation of expression of Ki-67 in squamous cell carcinoma of the glottis according to the degree of cell differentiation. Braz J Otorhinolaryngol 2014; 80: 290–295.
- Kim JYS, Kozlow JH, Mittal B, Moyer J, Olenecki T, Rodgers P. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol 2018; 78: 560–578.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol 2014; 32: 327–334.
- Nii T, Marumoto T, Kohara H, Yamaguchi S, Kawano H, Sasaki E, et al. Improved hematopoietic differentiation of primate embryonic stem cells by inhibition of the PI3K-AKT pathway under defined conditions. Exp Hematol 2015; 43: 901-911.e4.
- Tseng Y-H, Chang C-S, Liu T-Y, Kao S-Y, Chang K-W, Lin S-C. Areca nut extract treatment down-regulates involucrin in normal human oral keratinocyte through P13K/AKT activation. Oral Oncol 2007; 43: 670–679.
- Zhang W, Zeng W, Jiang A, He Z, Shen X, Dong X, et al. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: an analysis of the Global Burden of Disease Study 2019. Cancer Med 2021; 10: 4905–4922.
- Green AC, Olsen CM. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol 2017; 177: 373–381.
- Choi EH, Daruwalla A, Suh S, Leinonen H, Palczewski K. Retinoids in the visual cycle: role of the retinal G protein-coupled receptor. J Lipid Res 2021; 62: 100040.
- de Assis LVM, Lacerda JT, Moraes MN, Domínguez-Amorocho OA, Kinker GS, Mendes D, et al. Melanopsin (Opn4) is an oncogene in cutaneous melanoma. Commun Biol 2022; 5: 461.
- Stratigos A, Garbe C, Lebbe C, Malvehy J, del Marmol V, Pehamberger H, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer 2015; 51: 1989–2007.
- Pandey S, Søland TM, Bjerkli IH, Sand LP, Petersen FC, Costea DE, et al. Combined loss of expression of involucrin and cytokeratin 13 is associated with poor prognosis in squamous cell carcinoma of mobile tongue. Head Neck 2021; 43: 3374–3385.
- Roh V, Hiou-Feige A, Misetic V, Rivals J-P, Sponarova J, Teh M-T, et al. The transcription factor FOXM1 regulates the balance between proliferation and aberrant differentiation in head and neck squamous cell carcinoma. J Pathol 2020; 250: 107–119.
- Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, Mori-Estevez A-D, Sánchez-Acuña G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med Oral Patol Oral Cir Bucal 2013; 18: e174–e179.
- Juríková M, Danihel Ľ, Polák Š, Varga I. Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem 2016; 118: 544–552.