ORIGINAL REPORT
Serum Zinc Concentration in Patients with Alopecia Areata
Jovan LALOSEVIC1,2, Mirjana GAJIC-VELJIC1,2, Jelena LALOSEVIC MISOVIC3 and Milos NIKOLIC1,2
1Clinic of Dermatology and Venereology, University Clinical Center of Serbia, Belgrade, 2University of Belgrade School of Medicine, Belgrade and 3Department of Dermatovenereology, Clinical Hospital Center “Zvezdara”, Serbia
Alopecia areata is an autoimmune non-scarring disease in which the exact mechanism that induces loss of immune privilege is unknown. Zinc is important for DNA stability and repair mechanisms that are essential in maintaining normal hair growth. Zinc deficiency has been investigated as an important factor in many autoimmune diseases, and may have a possible role in the aetiopathogenesis of alopecia areata. This study included 32 patients with severe forms of alopecia areata, and 32 age- and sex-matched healthy controls. When comparing serum zinc levels in these 2 groups, statistically significantly lower zinc concentrations were found in the alopecia areata group (p = 0.017). Detected zinc deficiency was statistically more prevalent in patients with alopecia areata (p = 0.011). Evaluating patients with alopecia areata, a statistically significant negative correlation between serum zinc levels and severity of the disease was found (ρ = 0.006). The results indicate that zinc serum assessment is necessary in patients with alopecia areata. Low serum zinc levels were found to correlate with severity of alopecia areata. Given that most severe forms of alopecia areata are frequently most treatment-resistant, additional randomized control trials examining zinc supplementation are necessary to investigate its potential role in the restoration of hair follicles.
Key words: alopecia areata; zinc; Severity of Alopecia Tool.
SIGNIFICANCE
Alopecia areata is an autoimmune disease in which T-cells attack and damage hair follicles. The mechanism of this process is not fully understood. Zinc is known to be important for DNA stability, for normal hair growth, and in control of T-cells. This study found significantly lower levels of serum zinc in patients with alopecia areata than in healthy controls. Low levels of zinc are more pronounced in patients with long-lasting and more severe alopecia areata. The results of this study emphasize the importance of measurement of serum zinc levels in patients with alopecia areata, and provide a basis for further research into the importance of zinc supplementation in the treatment of alopecia areata with other modalities.
Citation: Acta Derm Venereol 2023; 103: adv13358. DOI https://doi.org/10.2340/actadv.v103.13358.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 6, 2023; Published: Oct 3, 2023
Corr: Milos Nikolic, Clinic of Dermatology and Venereology, University Clinical Center of Serbia, University of Belgrade School of Medicine, Belgrade, Serbia. E-mail: milos.nikolic@med.bg.ac.rs
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Alopecia areata (AA) is a common T-cell mediated autoimmune disease that affects hair follicles and results in non-scarring hair loss. The disease manifests as round or oval patches without hair, and can progress to a complete loss of hair on the scalp, as well as on other hairy parts of the body (1). AA affects all age groups, with no sex predominance (2). The lifetime risk of developing AA is 1.7%, while the prevalence in the general population is 0.2–2%, depending on the area of the world and the severity of the disease (3).
The aetiology of AA is unclear, but the collapse of immune privilege that leads to immune-derived destruction of the hair shaft presents the end result (4). The factors that induce such loss of immune privilege remain unclear, although genetic susceptibility, stress and environmental factors (viral infections, trace elements and micronutrients) play an important role (5).
Zinc is an essential micronutrient, which is required for more than 200 enzymatic reactions involved in many physiological processes (6). Regarding hair loss, zinc promotes the recovery of follicles through a series of activities, and its inhibition of endonuclease activity mediates the prevention of hair follicle regression. The matrix of the hair epithelium is one of the fastest proliferating and most sensitive tissues in the human body. Zinc is important for DNA stability and repair mechanisms that are essential in the maintenance of normal hair growth (7). Transient zinc deficiency is the main pathogenic factor in acrodermatitis enteropathica, a disease that, in addition to skin changes, leads to hair loss (8).
MATERIALS AND METHODS
A total of 64 participants were included in this prospective case-control study. Thirty-two participants were patients with different clinical forms of AA, primarily with more severe forms, where the area of the affected scalp skin was greater than 30%, who were hospitalized at the Clinic of Dermatology and Venereology of the University Clinical Center of Serbia, Belgrade, Serbia, from 1 January 2021 to 31 December 2022. Scalp involvement with AA was calculated based on the Severity of Alopecia Tool (SALT) score (9). Patients were divided into 2 groups, according to age: < 18 years and ≥ 18 years. The sex- and age-matched controls for patients younger than 18 years comprised paediatric patients treated at the same clinic who did not have AA or any other autoimmune or systemic disease, but a localized infection (viral warts, tinea corporis, or impetigo). The sex- and age-matched control group for the adults comprised healthy volunteers employed at the clinic.
In addition to the serum zinc level and SALT score, demographic characteristics (age and sex) were obtained, as well as the clinical form of AA, and the duration of the disease before examination at our clinic.
Clinical forms of AA were defined as: alopecia areata plurifocalis (AAP) (SALT 30–60%); alopecia areata subtotalis (AAS) (SALT 60–95%); AAP + ophiasis (AAP + OPH) in cases where patients had hair loss in occipital and retroauricular regions; alopecia totalis/universalis (AT/UN) (SALT 100%), with or without hair loss on other hairy parts of the body. For serum zinc analysis, 5 mL blood was taken using a vacutainer, left to clot, and then centrifuged to separate serum. Then, serum zinc concentrations were analysed by electrothermal atomic absorption spectrophotometry. As normal values, the laboratory specified the range 10.1–16.8 μmol/L for patients aged ≥ 11 years, and for those < 11 years, the reference range was 9.2–18.4 μmol/L. Statistical data analysis was performed using SPSS for Mac OS 23.0 program (IBM SPSS Statistics for Macintosh, Version 23.0. Armonk, NY: IBM Corp.) (χ2 test, Mann–Whitney U test, Spearman’s correlation, linear logistic regression). The normality of the distribution was tested with the Kolmogorov–Smirnov test.
RESULTS
The majority of the patients with AA were female, 19/32 (59.4%), predominantly children 20/32 (62.5%), median age 11 years (range 5–57 years). The majority of patients had severe forms of AA, with SALT ≥ 30 (AT/UN 34.4%, 11/32; AAS 31.3%, 10/32; AAP + OPH 9.4%, 3/32), while only 25%, 8/32 patients, had AAP. The mean SALT for the patients with AA was 70.6% ± 25.9.
The median serum zinc level in patients with AA was 9.72 μmol/L (range 6.06–14.71), while the control group had a median zinc level of 10.70 μmol/L (range 8.52–14.83). Comparing the 2 groups, statistically significantly lower concentrations of serum zinc were found in the AA group, p = 0.017 (Fig. 1).
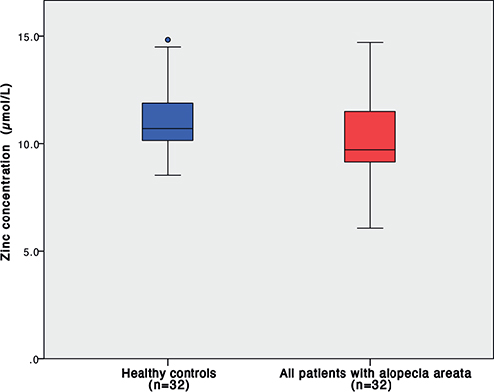
Fig. 1. Serum zinc levels in patients with alopecia areata and healthy controls; p = 0.017.
Furthermore, comparing adults and children with AA with their respective control groups revealed that, even though concentrations were lower in both groups of patients with AA, statistically significantly lower zinc concentrations were found in children with AA (Fig. 2).
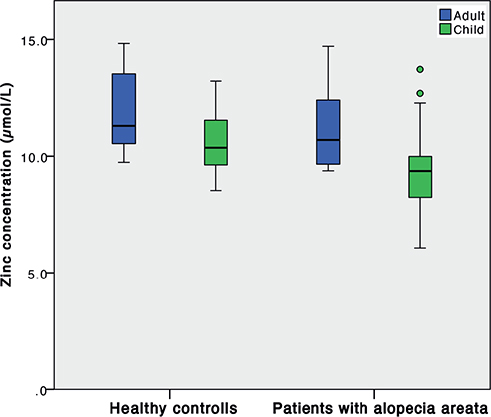
Fig. 2. Serum zinc concentrations in patients with alopecia areata and healthy controls, adults (blue) and children (green); p = 0.236 and p = 0.024, respectively.
There was a statistically significant negative correlation between the severity of AA (SALT score) and zinc serum concentrations (Fig. 3); ρ = 0.006.
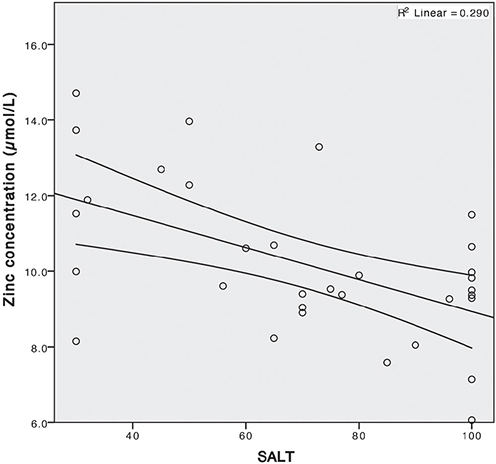
Fig. 3. Negative correlation of serum zinc concentration and severity of alopecia areata (measured by Severity of Alopecia Tool (SALT) score); ρ = 0.006, R = 0.290.
When normal serum zinc concentration values, as defined by the laboratory, were taken into consideration, a significantly higher number of patients with zinc deficiency was found in the AA group in comparison with healthy controls: 43.8% (14/32) vs 12.5% (4/32), respectively, p = 0.011. Zinc deficiency was significantly more expressed in severe clinical forms of AA (AAS 6/10 and AT/UN 7/11); p = 0.039. Similar to the results regarding serum zinc concentrations, these results showed a high prevalence of zinc deficiency in both the adult and children groups with AA: 41.7% (5/12) and 45.0% (9/20), respectively. In healthy controls, low zinc concentration prevalence was inferior to that in patients with AA (zinc deficiency was found in 8.3% (1/12) children and in 15.0% (3/20) adults), the difference was not statistically significant (p = 0.082 and 0.155, respectively).
DISCUSSION
Hair matrix epithelium is one of the most rapidly proliferating and most sensitive tissues. Zinc is important for DNA stability and repair, making it essential for normal hair growth (10). The exact mechanisms by which zinc affects hair loss is still not clarified, although metalloenzymes containing zinc have a potential role in the regulation of hair growth. So-called zinc fingers are small protein structural motifs for many transcription factors that regulate hair growth via the hedgehog signalling pathway; zinc is also a catagen inhibitor through its inhibitory effect on the apoptosis-inducing endonuclease (11).
Several studies have investigated zinc levels in patients with AA (Table I). The current study found a statistically highly significantly lower serum zinc concentration in patients with AA than in controls. This is in accordance with the results of studies by Abdel Fattah et al. (12), Bhat et al. (13) and Kil et al (7). Different results were published by Mussalo-Rauhamaa et al. (14), who found no differences in concentrations of trace elements, including zinc, in patients with AA compared with controls. Possible reasons for such discrepancies may be related to different zinc assessment methodology, sample size, or population variation. A study by Dastgheib et al. investigated trace element levels in both serum and hair, comparing healthy controls and patients with AA (15). In contrast to the current findings, they did not find significant differences in trace element levels, including zinc, when comparing patients with AA and controls. The difference could be due to the fact that patients included in Dastgheib et al.’s study were mild to moderate cases, with no patients with AA totalis or AA universalis enrolled in the study (15). Nevertheless, a study by Amirnia et al. (16) that also examined hair and serum zinc levels in patients with AA, found significantly lower levels compared with age-/sex-matched controls, in agreement with the current results.
| Study | Year | Study type | Study population | Sample size | Results |
| Abdel Fattah et al. (12) | 2016 | Case-control (hospital-based) | Sex: 39M/11F; Age: 27 ± 9.5 years Country: Egypt |
100 (50 AA cases and 50 sex- and age-matched controls) | Significantly lower serum Zn in AA patients vs controls |
| Bhat et al. (13) | 2009 | Case-control (hospital-based) | Sex: 34M/16F; Age: 27.3 years Country: India |
100 (50 AA cases and 50 healthy sex- and age- matched controls | Significantly lower serum Zn in AA patients vs controls |
| Mussalo-Rauhamaa et al. (14) | 1986 | Case-control (hospital-based) | Sex: 8M/19F; Age: 29 ± 11 years Country: Finland |
27 AA cases compared with normal Finnish population reference values | No difference in serum zinc levels in AA vs normal population. Compared AA cases to serum zinc values from the normal Finnish population |
| Dastgheib et al. (15) | 2014 | Case-control (not described) | Sex: 16F; Age: 26.6 ± 8.5 years Country: Iran |
43 (16 AA cases and 27 sex- and age-matched healthy controls) | No difference in serum zinc levels or hair zinc levels in AA patients vs controls |
| Amirnia et al. (16) | 2013 | Case-control (hospital-based) | Sex: 3M/24F; Age: 66.3 ± 9.9 years Country: Iran |
54 (27 AA cases and 27 healthy sex- and age- matched controls) | Significantly lower serum and hair Zn in AA patients vs controls |
| Kil et al. (7) | 2013 | Case-control (hospital-based) | Sex: 44M/50F; Age: 37.1 ± 14.9 years Country: Korea |
126 (94 AA cases and 32 healthy controls) | Significantly lower serum Zn in AA patients vs controls |
| Current study | 2023 | Case-control (hospital-based) | Sex: 13M/19F; Age: 18.1 ± 13.4 years, Children/Adults: 20/12 Country: Serbia |
64 (32 AA cases and 32 healthy sex- and age-matched controls) | Significantly lower serum Zn in AA patients vs controls |
Abdel Fattah et al. (12) concluded that patients with long-lasting AA, and patients with higher SALT scores are more prone to have low serum zinc. The current results confirm such findings, emphasizing the potential role of zinc in the aetiopathogenesis of more severe and more treatment-resistant forms of AA.
The current results also show that zinc deficiency, and not only lower levels, are more pronounced in patients with AA than in healthy controls. This finding confirms the previously published results and meta-analysis, stating that zinc deficiency can be associated with susceptibility to AA (17), but now confirms it in a new population (South-Eastern European).
However, the true aetiology of AA is yet to be elucidated. Given that zinc has immunomodulatory effects protecting high cellular turnover tissue from oxidative stress, its role in maintaining a normal hair cycle in hair disorders is critical (7, 18). T cell function is highly zinc-dependent and zinc-regulated. Zinc deficiency leads to malfunctions and altered T cell differentiation, resulting in thymic atrophy, and an unbalanced immune response, creating an immunological milieu promoting autoimmune diseases (19). A special subset of T cells, named regulatory T cells (Treg), is responsible for the downregulation of immune reactions, such as inflammation. Whereas effector T cells, such as Th17 cells, produce molecules inducing inflammations, their counterparts, Treg cells, suppress effector T cell mediated immune reactions by producing anti-inflammatory cytokines. Treg cells are important to keep effector T cells in check, preventing immune reactions from getting out of hand (20).
The results of the current study show that children with AA have significantly lower zinc concentrations than adults with AA, compared with healthy controls. This finding could be due to a smaller sample size in the adult group. To the best of our knowledge, no studies have indicated zinc as an associated factor in childhood AA (Table I).
The current study found that serum zinc concentrations correlate inversely with AA severity, and since the severest AA forms most commonly are treatment-resistant, additional supplementation with zinc could potentially be beneficial in patients with AA. There are 5 studies that have investigated zinc as a treatment option for AA, 3 of which were placebo controlled (21, 22). In 2 studies there was a positive effect of zinc supplementation on hair regrowth in patients with AA (22, 23), while 1 study showed no significant difference compared with controls (24). The methodology of these studies differ with regard to the population, age, and doses of zinc supplementation.
The observed association between low serum zinc concentration and AA does not automatically imply that zinc plays a role in the pathogenesis of AA; however, we can conclude that assessing the serum zinc level is necessary in evaluating patients with AA, especially in children. Further double-blind, placebo-controlled studies are necessary to determine whether zinc supplementation, together with its exact form, dose and duration, might be important in the treatment of AA.
ACKNOWLEDGEMENT
This study was supported by the Ministry of Education and Science of the Republic of Serbia (Grant 175065; M Nikolic, M Gajic- Veljic).
REFERENCES
- Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primer 2017; 3: 17011.
- Juarez Rendón K, Rivera G, Reyes-López M, Garcia-Ortiz J, Bocanegra-Garcia V, Guardiola I, et al. Alopecia areata. Current situation and perspectives. Arch Argent Pediatr 2017; 115: e404–411.
- Lee HH, Gwillim E, Patel KR, Hua T, Rastogi S, Ibler E, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol 2020; 82: 675–682.
- Azzawi S, Penzi LR, Senna MM. Immune privilege collapse and alopecia development: is stress a factor. Skin Appendage Disord 2018; 4: 236–244.
- Suchonwanit P, Kositkuljorn C, Pomsoong C. Alopecia areata: an autoimmune disease of multiple players. ImmunoTargets Ther 2021; 10: 299–312.
- Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: a review of the evidence. Br J Nutr 2008; 99: S14–23.
- Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol 2013; 25: 405.
- Kumar S, Thakur V, Choudhary R, Vinay K. Acrodermatitis enteropathica. J Pediatr 2020; 220: 258–259.
- Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. Alopecia areata investigational assessment guidelines–Part II. J Am Acad Dermatol 2004; 51: 440–447.
- Lee SH, Seo SH, Lee DH, Pi LQ, Lee WS, Choi KY. Targeting of CXXC5 by a competing peptide stimulates hair regrowth and wound-induced hair neogenesis. J Invest Dermatol 2017; 137: 2260–2269.
- Fantauzzo KA, Bazzi H, Jahoda CAB, Christiano AM. Dynamic expression of the zinc-finger transcription factor Trps1 during hair follicle morphogenesis and cycling. Gene Expr Patterns 2008; 8: 51–57.
- Abdel Fattah NSA, Atef MM, Al-Qaradaghi SMQ. Evaluation of serum zinc level in patients with newly diagnosed and resistant alopecia areata. Int J Dermatol 2016; 55: 24–29.
- Bhat Y, Manzoor S, Khan A, Qayoom S. Trace element levels in alopecia areata. Indian J Dermatol Venereol Leprol 2009; 75: 29.
- Mussalo-Rauhamaa H, Lakomaa EL, Kianto U, Lehto J. Element concentrations in serum, erythrocytes, hair and urine of alopecia patients. Acta Derm Venereol 1986; 66: 103–109.
- Dastgheib L, Mostafavi-pour Z, Abdorazagh AA, Khoshdel Z, Sadati MS, Ahrari I, et al. Comparison of Zn, Cu, and Fe content in hair and serum in alopecia areata patients with normal Group. Dermatol Res Pract 2014; 2014: 784863.
- Amirnia M, Sinafar S, Sinafar H, Nuri M, Sadeghi AT. Assessment of zinc and copper contents in the hair and serum and also superoxide dismutase, glutathion peroxidase and malondi aldehyde in serum in androgenetic alopecia and alopecia areata. Life Sci J 2013; 10: 204–209.
- Jin W, Zheng H, Shan B, Wu Y. Changes of serum trace elements level in patients with alopecia areata: a meta-analysis. J Dermatol 2017; 44: 588–591.
- Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 2018; 68: 19–31.
- Rosenkranz E, Maywald M, Hilgers RD, Brieger A, Clarner T, Kipp M, et al. Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J Nutr Biochem 2016; 29: 116–123.
- Waśkiel-Burnat A, Osińska M, Salińska A, Blicharz L, Goldust M, Olszewska M, et al. The role of serum Th1, Th2, and Th17 cytokines in patients with alopecia areata: clinical implications. Cells 2021; 10: 3397.
- Thompson JM, Mirza MA, Park MK, Qureshi AA, Cho E. The role of micronutrients in alopecia areata: a review. Am J Clin Dermatol 2017; 18: 663–679.
- Sharquie K, Noaimi A, Shwail E. Oral zinc sulphate in treatment of alopecia areata (double blind; cross-over study). J Clin Exp Dermatol Res 2012; 3: 2
- Camacho FM, García-Hernández MJ. Zinc aspartate, biotin, and clobetasol propionate in the treatment of alopecia areata in childhood. Pediatr Dermatol 1999; 16: 336–338.
- Ead RD. Oral zinc sulphate in alopecia areata – a double blind trial. Br J Dermatol 1981; 104: 483–484.