SHORT COMMUNICATION
Rapid Diagnosis of Mycobacterium marinum Infection on Both Hands using a Combination of Skin Trephination and Metagenomic Next-generation Sequencing
Chaochao JI1, Jiaqi LIU2, Yue MOU3, Wenhao CHENG3 and Wenlong HU1,3,4*
1Lianyungang Clinical College of Nanjing Medical University/The First People’s Hospital of Lianyungang, Lianyungang, Jiangsu, 2Graduate School, Jinzhou Medical University, Jinzhou, Liaoning, 3Department of Dermatology, The Affiliated Lianyungang Hospital of Xuzhou Medical University/The First People’s Hospital of Lianyungang and 4Department of Dermatology, The First Affiliated Hospital of Kangda College of Nanjing Medical University, Lianyungang, Jiangsu, China. *E-mail: lyghuwl@163.com
Citation: Acta Derm Venereol 2023; 103: adv13360. DOI https://doi.org/10.2340/actadv.v103.13360.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 24, 2023; Published: Sep 20, 2023
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Mycobacterium marinum infection, also known as fish-tank granuloma, swimming pool granuloma, or aquarium granuloma, is caused by a non-tuberculous mycobacterium (1). Diagnosis of the disease is traditionally based on a clear history of exposure, in addition to the presence of clinical lesions, the results of histopathology and acid-fast staining, and identification of the pathogen in a culture. Identification of the pathogen is important for a clear diagnosis, although M. marinum is slow-growing and, therefore, traditional laboratory culture identification may be very time-consuming. In recent years, clinicians have been able to rapidly diagnose M. marinum infections in clinical practice using metagenomic next-generation sequencing (mNGS) (2). We describe here the use of mNGS to rapidly diagnose a patient with M. marinum infection on both hands. Clinically, bilateral infections with M. marinum, such as occurred in this patient, are extremely rare.
CASE REPORT
A 73-year-old Chinese woman presented with red eruptions on the skin of both hands for 1-year. She was a housewife who often cooked fish and was not aware of her trauma history, and could not remember if the lesions on both hands occurred at the same time. The patient was diagnosed with dermatitis or eczema at several hospitals and was treated with topical corticosteroids, although this did not result in improvement in the skin lesions. Subsequently, she presented to the outpatient department of the First People’s Hospital of Lianyungang (Lianyungang, Jiangsu, China) for treatment. Dermatological examination showed dark-red patches on the dorsal proximal phalanx regions of the left thumb and right index finger (Fig. 1A). No fever or other systematic symptoms were observed. Skin trephination was performed to obtain skin tissue, which was sent for testing using mNGS (BGI-Nanjing; Nanjing, Jiangsu, China). Human host sequences were eliminated. The remaining data were compared with BGI’s 4 microbial genome databases, which included 10,989 bacteria, 5,050 viruses, 1,179 fungi and 282 parasites. Within 48 h of receipt of the sample, mNGS detected sequence reads of M. marinum in the patient’s skin tissue. Based on the detection report, only M. marinum was the most likely pathogenic pathogen. The remaining bacteria found by mNGS were considered to be human colonizers or contaminating bacteria in the sampling and testing process (Table SI). In addition, mNGS also detected small amounts of fungi and parasites (Table SII), which we do not consider sufficient to cause disease. After analysing the identification report of mNGS, a diagnosis of M. marinum infection was made. The patient was advised to start rifampicin therapy. Mycobacterial culture was positive (Fig. 1B) and M. marinum was identified with a PCR after 1 month, which further confirmed the diagnosis of M. marinum infection. After 5 months of oral administration of rifampicin, the patient’s rash subsided completely and did not relapse during the following 6 months of follow-up.
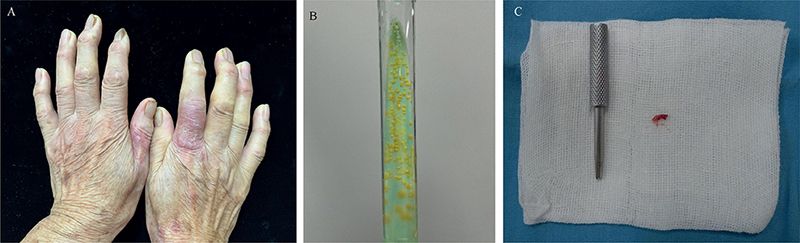
Fig. 1. (A) Dark-red patches on the dorsal proximal phalanx regions of the left thumb and right index finger. (B) Mycobacterial culture was positive with yellow colonies. (C) Skin trephination to collect very small amounts of tissue.
DISCUSSION
The majority of M. marinum infections usually occur several weeks after injured skin comes into direct or indirect contact with contaminated water or aquatic animals, such as fish or oysters (1, 3). The disease usually presents as a slow-growing, painless, isolated skin lesion at the site of inoculation, with a variety of skin manifestations developing at the site of the lesion (3). Disseminated infections are rare and occur mainly in individuals with either low immunity, immunosuppressant use, or immune deficiency (1, 3).
The doctors who treated the patient before she was admitted to our hospital did not consider that she was infected with M. marinum and misdiagnosed dermatitis or eczema. This probably occurred because the patient had a bilateral infection, while patients almost always have a unilateral infection. Although bilateral infection with M. marinum is extremely rare clinically, it is possible for dermatologists to encounter such patients, and such cases have been reported in earlier studies (4, 5). We hypothesized that the bilateral infection occurred in this patient because both her hands were infected coincidentally at the same time, and that the patient did not have a haematogenous spread of M. marinum.
The patient was biopsied using a skin trephination to collect very small amounts of tissue that were adequate for mNGS (Fig. 1C). Trephination has the advantages of a small wound, simple operation, and more rapid recovery after surgery compared with biopsy performed with a scalpel. The majority of pathogens grow slowly or cannot be cultured, although mNGS avoids this limitation by rapidly detecting nucleic acid sequences of infectious pathogens without the need for isolation and culture (6). In theory, the unique identification of almost all microorganisms could be realized based on specific nucleic acid sequences (6). Rapid identification of the infected pathogen is conducive to guiding the clinical targeted use of antibiotics and assisting precise treatment of the infection. The combination of skin trephination and mNGS can therefore be used as an effective method for rapid diagnosis of rare pathogen infections and is worthy of extensive clinical application.
REFERENCES
- Lewis FMT, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis 2003; 37: 390–397.
- Xing F, Lo S, Ma Y, Ip JD, Chan WM, Zhou M, et al. Rapid diagnosis of mycobacterium marinum infection by next-generation sequencing: a case report. Front Med 2022; 9: 824122.
- Khan UM, Rittenberg A. Fisherman’s dilemma: disseminated mycobacterium marinum in an immunosuppressed patient. Am J Med 2020; 133: e549–e551.
- Su Q, Wang F. Painful nodules on the arms. N Engl J Med 2021; 384: e41.
- Liu J, Yao Q, Cheng W, Ren H, Hu W. Mycobacterium marinum infection on both hands masquerading as ‘eczema’. Am J Med 2023; 136: e5–e6.
- Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol 2019; 14: 319–338.