REVIEW ARTICLE
Atopic Dermatitis in the Elderly Population
Martina MAURELLI1, Andrea CHIRICOZZI2,3, Ketty PERIS2,3, Paolo GISONDI1 and Giampiero GIROLOMONI1
1Section of Dermatology and Venereology, Department of Medicine, University of Verona, Verona, 2Dermatology, Department of Medical Science and Surgery, Fondazione Policlinico Universitario A. Gemelli IRCCS and 3Department of Dermatology, Catholic University of the Sacred Heart, Rome, Italy
Atopic dermatitis is a common inflammatory disease with a chronic and relapsing course. Although considered a childhood disease, it is now evident that atopic dermatitis is also common in adulthood and in the elderly population. Atopic dermatitis typically manifests with bilateral and symmetrical eczematous lesions on the face, trunk and skin folds. Itch is invariably present and may be very severe, markedly affecting daily life and sleep. In older adults, atopic dermatitis may have a high level of impact on quality of life, frequently burdening an already complex comorbid situation. The full assessment of disease burden (localizations, itch severity, sleep alterations, impact on quality of life, disease history, comorbidities) is crucial to identify the most appropriate treatment. In many cases, moderate-to-severe atopic dermatitis in the elderly population can be successfully and safely treated with biological agents inhibiting the interleukin-4/-13 pathway, whereas the use of Janus kinase inhibitors may pose concerns about the safety profile.
Key words: atopic dermatitis in elderly; atopic dermatitis; elderly; older patients; treatment.
SIGNIFICANCE
Atopic dermatitis is a common inflammatory and chronic disease, which is common in children, adulthood and the elderly population. Atopic dermatitis manifests typically with bilateral and symmetrical eczematous lesions on the face, trunk and skin folds. Itch is invariably present and may be very severe. In older adults, atopic dermatitis may have a high level of impact on quality of life and comorbidities. Currently available drugs offer the possibility of an effective and safe treatment.
Citation: Acta Derm Venereol 2023; 103: adv13363. DOI https://doi.org/10.2340/actadv.v103.13363.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 11, 2023; Published: Dec 14, 2023
Corr: Martina Maurelli, Section of Dermatology and Venereology, Department of Medicine, University of Verona. Piazzale A. Stefani 1, IT-37126 Verona, Italy. E-mail: maurelli.martina@gmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
A topic dermatitis (AD) is a common inflammatory disease with a chronic and relapsing course, which typically manifests with dry skin and symmetrically distributed eczematous dermatitis. Itch is invariably present and, at times, very severe, markedly affecting daily life and sleep. The pathogenesis of AD involves skin barrier defects, T-helper type 2 (Th2) dominated skin inflammation and skin hyper-responsiveness to inflammatory insults (1, 2). Genetic predisposition is relevant and is often the first step in the development of these atopic diseases, such as bronchial asthma and/or allergic rhino-conjunctivitis. Although AD was considered a typical childhood disease, recent epidemiological studies have revealed that it is also common in adulthood and in elderhood (3, 4). In older adults, AD may have a high level of impact on quality of life and disease burden, frequently adding to an already complex comorbid situation. In addition, treatment of moderate-to-severe AD in elderly people may pose concerns in relation to comorbid diseases and concomitant medications (2). This narrative review discusses the principal clinical, diagnostic, immune-pathogenic aspects, and therapeutic implications of AD in the elderly population (65 years or older).
CLINICAL MANIFESTATIONS OF ATOPIC DERMATITIS IN THE ELDERLY POPULATION
Several AD phenotypes have been described according to lesion distribution, such as flexural, head and neck, periorificial, or the predominant clinical feature, such as nummular eczema, prurigo nodularis, lichenified dermatitis, and follicular/papular dermatitis (5).
Epidemiological data on AD in older adults are sparse, as few studies include this population. Physician-identified AD was estimated to affect 11.6% of older adults in the UK, with an annual prevalence of 7.0–9.3% among older adults aged 75–99 years (6), and a higher prevalence in males (3, 6–8). The percentage of patients with moderate-to-severe AD was higher in older adults compared with younger patients (6). Regarding the onset and clinical course, elderly AD can be distinguished in late onset, recurrent with a history of childhood AD, and continuation and/or recurrence of adolescent/adult AD (4). Approximately one-third of adult/elderly cases represent new-onset AD, although a recall bias for milder disease occurring in childhood should be taken into account.
Skin manifestations of AD in elderly people match with those observed in adolescents and adults, with eczematous dermatitis on the face and neck, as well as on the folds of both upper and lower extremities (Figs 1 and 2). Lesions are vesicular and exudative in the acute phase and hyperkeratotic and desquamative in the chronic phase. The involvement of the trunk is very common compared with childhood AD (9). Eczema lesions tend to be bilateral with a symmetrical distribution, which is an important consideration in the differential diagnosis with contact dermatitis. Lichenified lesions with or without papules and nodules are frequently observed in the chronic phase, and the reverse sign of lichenified eczema around unaffected folds of the elbows and knees is common (8, 9). Atopic red face (facial erythema), Hertoghe’s sign (loss of the lateral eyebrows), Dennie-Morgan infraorbital folds, dirty neck and follicular lichenified papules can be observed (4). Nummular or discoid eczema, clinical variants of prurigo (papular and nodular) and erythrodermic rash may also be observed at a higher frequency compared with younger patients. Clinical manifestations in older adults (> 65 years) have been associated with a significantly lower incidence of active lesions at the face and scalp, marginally significant lower incidence of flexural (including elbow and knee folds) lesions, and a higher incidence of lesions on the buttocks or genitals (8, 9).

Fig. 1. Atopic dermatitis of the face in older women (age range 82–94 years). Lesions are symmetrical and more severe in the periorbital region. Written permission to publish the photos was obtained from the patients.
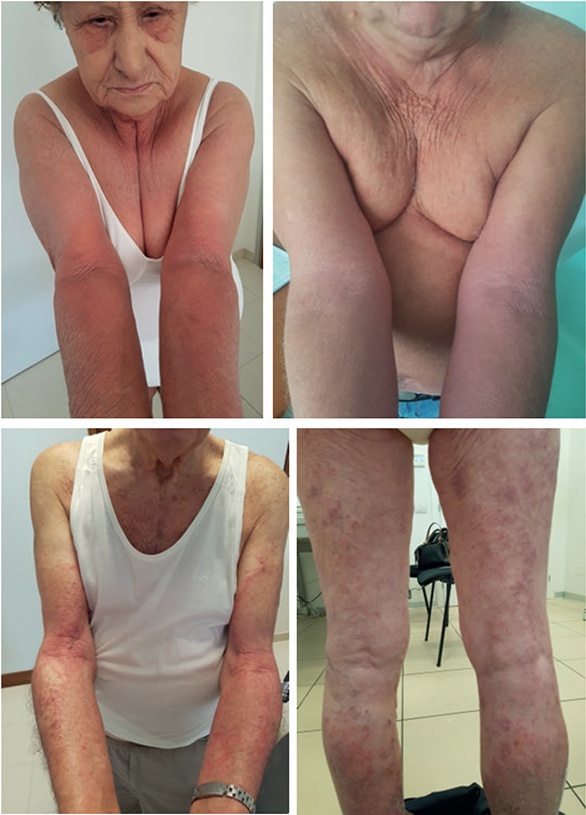
Fig. 2. Atopic dermatitis affecting the face, volar surface of upper arms and legs in older patients (age range 78–87 years). Lesions are typically symmetrically distributed. Written permission to publish the photos was obtained from the patients.
Based on positivity to skin-prick tests or serum IgE levels, 2 types of AD may be identified: an IgE allergic (extrinsic) type associated with IgE-mediated sensitization to environmental allergens and high levels of total IgE (more than 200 or 400 IU/L) and allergen-specific IgE (positive results in skin-prick test and/or serum-specific IgE evaluations); and a non-IgE-allergic (intrinsic) type with normal levels of total IgE and lack of sensitization to environmental allergens (1, 5). The most common environmental allergens in IgE-allergic elderly patients with AD are house dust mites, followed by pollens (4). Notably, patients might have normal serum total IgE, albeit positivity for allergen-specific IgE may be detected (8, 10, 11). Therefore, the presence of elevated serum IgE or the presence of specific IgE in the skin or serum does not represent a major diagnostic criterion for AD. Indeed, allergen-specific IgE responses have no relevant role in disease onset and/or perpetuation in most cases, and desensitization is only marginally effective in a few cases (11–13). Itch is present in all phases of AD and may be very severe, particularly at night, thus affecting the quantity and quality of sleep. As a consequence of sleep deprivation, daily activities can be markedly affected, even in patients with mild-moderate disease (14–16). Dry skin is invariably present and may worsen with increasing age (11).
AETIOPATHOGENESIS OF ATOPIC DERMATITIS
The hallmark of AD is the epidermal barrier dysfunction associated with type 2 inflammation in the skin (1). Epidermal barrier defects favour increased transepidermal water loss (skin xerosis) and increased skin penetration of allergens, microorganisms, and irritants. Mutations in the filaggrin protein gene (as well as other protein constituents of terminally differentiated keratinocytes), uncontrolled activity of epidermal proteases and reduction in stratum corneum lipids (ceramides) are the main drivers of barrier dysfunction. Decreased stratum corneum lipids, especially ceramides, appear to be a major aetiological factor for dryness of elderly skin (4, 10). Barrier impairment represents the initial step of the atopic march towards allergic respiratory diseases. Barrier defects facilitate the entry of irritants (sweat, inappropriate clothing), pathogens (Staphylococcus aureus) and allergens (house dust mites, pollens, fungi), which may also promote the expression of pro-inflammatory cytokines and chemokines in the epidermis, with fostering of type 2 responses and further decrease in filaggrin generation. Reductions in barrier functions associated with ageing might thus increase susceptibility to environmental irritants, pathogens and allergens that can lead to systemic sensitization, and/or favour type 2 immune responses in elderly AD. A decreased immunohistochemical expression of filaggrin has also been observed in elderly xerosis (3, 11).
AD skin is dominated by preferential activation of type 2 inflammatory cells, in particular Th2 cells producing interleukin (IL)-4, IL-5, IL-13 and IL-31 (1, 17, 18). Multiple immune pathways contribute to the development of the atopic phenotype, acting in parallel (Th22, Th17, or Th1) or enhancing the type 2 signal (Th9 and Th21) (17). Th1, and to some extent Th17 cells increase in the chronic lesions but do not outnumber Th2 cells. Th2 cytokines, IL-4 and IL-13, stimulate B cells to produce IgE antibodies and promote IgE-induced upregulation of the high-affinity IgE receptor (FcεRI) on mast cells and dendritic cells, and the IL-5 induces peripheral eosinophil activation. Th2 and Th22 cytokines (IL-4, IL-13, IL-22) contribute to the impaired expression of barrier proteins (filaggrin) and barrier dysfunction of lesional skin (17). Th2 cytokines, in particular IL-4, IL-13 and IL-31, are also the major inducers of itch with a contribution of thymic stromal lymphopoietin, periostin and proteases (14). IL-22 induces epidermal hyperplasia of chronic lesions in patients with AD. In patients with extrinsic type AD, high numbers of IgE-bearing cells, including mast cells, dermal dendritic cells, inflammatory dendritic epidermal cells, and Langerhans cells can be detected in lesional AD skin (19). Adults, and also elderly patients, affected by AD, show further increase in Th17/Th22 (S100A12, IL-20) and Th2 (CCL13, CCL17, OSM, IL-1RL1/IL-33R, IL-4, IL-4R) related markers vs younger patients with AD, whereas IL-4R and IL-4 were the proteins showing up-regulation vs controls, exclusively in the adult AD age group (20).
Peripheral blood cytokine concentrations demonstrated the dominance of a Th2 cytokine profile (IL-4, IL-5, and IL-13) in older patients with IgE-allergic AD, whereas higher levels of interferon (IFN)-γ have been measured in older patients with AD and low serum total IgE levels. In addition, elderly patients with AD tended to show some immune skewing with decreased Th2 and Th22 cytokines and increased Th1 cytokines in lesional skin compared with patients with adult AD, highlighting a more mixed activation of multiple immune pathways in elderly compared with younger subjects with AD (19–21). The age of onset may also affect the immune profile in adulthood AD, as paediatric-onset AD showed greater inflammation in lesional skin, with more prominent expression of Th2/Th17/Th22 markers, revealing a different endotype compared with adult-onset AD, which was characterized by some more Th1-skewing (22). Overall, Th2 immunity and inflammation also appear to be crucial in the pathogenesis of AD in the elderly population, as selectively blocking Th2 cytokines is very effective in treatment of elderly AD. Skin proteins released by scratching, foods, environmental airborne allergens and products of infectious pathogens can induce allergen-specific IgE antibodies, but the role of allergen-specific responses is unclear in elderly patients as it is in younger patients. AD skin is also characterized by defective cutaneous innate immunity to microorganisms and the production of antimicrobial peptides is decreased in AD skin (1). These and other defects in innate immunity mechanisms favour skin infection, particularly by S. aureus and herpes viruses.
ATOPIC DERMATITIS DIAGNOSIS
Diagnosis of AD is, in general, clinical (5). An eczematous dermatitis affecting the face and neck area, the skin folds and/or the trunk with a bilateral and symmetrical distribution is key. In addition, a personal and/or family history of atopic disorders and elevated serum levels of total IgE and allergen-specific IgEs in response to environmental allergens or peripheral blood eosinophilia can support the diagnosis, although it is not necessary. Follow-up of the clinical course, analysis of the diagnostic features of AD using standardized criteria, and exclusion of other pruritic skin conditions (asteatotic dermatitis, elderly pruritus and chronic prurigo) are also important. Several diagnostic criteria for diagnosis of AD in adult and elderly patients have been developed with variable specificity and sensitivity. Recently issued diagnostic criteria have simplified the diagnostic approach compared with the more complex original Hanifin & Rajka criteria. In particular, Chinese criteria for adult and adolescent AD point to the mandatory presence of persistent or recurrent symmetrical eczema for more than 6 months. In addition, one or more of the following elements are necessary for the diagnosis: personal and/or family history of atopic diseases; elevated total serum IgE levels and/or positive allergen-specific IgE and/or eosinophilia (23, 24) . Japanese criteria consider AD as a chronic disease characterized by fluctuating phases of remitting and relapsing eczema with pruritus as a primary symptom. Japanese guidelines also consider the symmetrical distribution of lesions as a key diagnostic criterion for the diagnosis. Minor criteria include personal or family history of AD and other atopic diseases and overproduction of IgE antibodies. (25). These criteria have a specificity and sensitivity higher than the classical Hanifin & Rajka criteria and have also been applied to the elderly population. There are no recent diagnostic criteria applied to elderly or adult AD in Caucasians, but there is no strong reason to posit that Caucasian patients behave clinically differently compared with Asian subjects.
The main differential diagnosis of AD in adults and elderly patients include contact dermatitis, particularly in localized forms (hand dermatitis), seborrhoeic dermatitis, psoriasis, scabies, tinea, drug eruptions, and, most importantly, cutaneous T cell lymphoma (Table I). Exclusion of other disorders may require skin biopsy for histological examination, immunohistochemistry and T cell receptor gene rearrangement studies, search for dermatophytes by direct microscopy or culture, and Sarcoptes scabiei by dermoscopy. Histology of AD shows the features of acute or chronic eczema and does not distinguish AD from contact dermatitis (5). It should also be kept in mind that the above-mentioned disorders may occur in patients with past history or current AD. The major differences and similarities among childhood, adulthood and elderhood AD have been summarized in Table II.
| Childhood | Adulthood | Elderhood | |
| Clinical features | Face, extensor surfaces of the limbs and flexural involvement and trunk (less frequent) in older children | Face and neck, trunk as well as folds of both upper and lower extremities | Lower incidence on the face and scalp, marginally significant lower incidence of flexural higher incidence of lesions on the buttocks or genitals |
| Extracutaneous manifestations of atopy | Frequent | Less frequent | |
| Immune profile | Dominant type 2 inflammation (Th2) Elevated total IgE |
Th2 associated with Th17, Th22, and Th1 Possibly elevated total IgE |
Mixed immune pathways with lower Th2 and higher Th22 and Th1 contribution Less commonly elevated total IgE |
| Skin barrier impairment | Present at any age | ||
| Filaggrin mutation | Frequently detected | Only in early-onset AD | |
| Suitable therapeutic options | Dupilumab Tralokinumab* Cyclosporine JAK inhibitors* |
Cyclosporine Dupilumab Tralokinumab Methotrexate Azathioprine JAK inhibitors |
Dupilumab Tralokinumab Methotrexate azathioprine JAK inhibitors |
| *Adolescents (> 12 year-old). JAK: Janus kinase; Th2: T-helper type 2; IgE: immunoglobulin E. | |||
TREATMENT OF ATOPIC DERMATITIS IN THE ELDERLY POPULATION
Xerosis is a typical feature of ageing skin in older adults, and frequent washing and showering may lead to the worsening of dry skin. Therefore, gentle washing is recommended, and rubbing with a towel should be avoided. Moisturizers should be applied immediately after bathing and showering before the skin is completely dry. Moisturizers should be used on a widely on the skin surfaces, both lesional and non-lesional skin, and their use should be continued after the resolution of skin lesions (5). Several topical and systemic treatments are available for AD. Administration route, dosage, efficacy and safety profile and contraindications are detailed in very recent publications (2, 26). In addition, many new and emerging treatments are under investigation (27). Mild-to-moderate cases of AD can be easily controlled by topical treatments, which includes calcineurin inhibitors (tacrolimus and pimecrolimus) and corticosteroids. Phosphodiesterase 4 and Janus kinase (JAK) inhibitors are already approved outside Europe (26, 27). In addition, for mild-to-moderate chronic AD, phototherapy with narrowband ultraviolet B or UVA1 may be considered a valid therapeutic option, particularly in the treatment of elderly AD in chronic phase (26).
Treatment for moderate-to-severe AD requires systemic drugs, and their use may be challenging in elderly patients (28, 29). In particular, the use of conventional systemic immunosuppressive drugs (e.g. methotrexate, cyclosporine, azathioprine, mycophenolate) needs to take into account the frequent metabolic comorbidities occurring in the elderly population, including arterial hypertension, atherosclerotic disease, diabetes, dyslipidaemia and osteoporosis, and the progressive deterioration of kidney and liver functions. In addition, patients’ cognitive ability, functional independence, ability to self-administer drugs, the concomitant treatments (polypharmacy) and the possible harmful drug interactions need to be considered. Short courses of oral steroids (2–4 weeks, 20–40 mg prednisolone) may be effectively used for acute flares (2). Methotrexate, azathioprine, and cyclosporine are frequently contraindicated and may be used with caution for various conditions and polypharmacy. Methotrexate interacts with other hepatotoxic drugs or medications such as sulphamethoxazole, anti-inflammatory drugs, and allopurinol (2). Azathioprine also poses concerns about liver and bone marrow toxicity, and usually has a long-term latency before showing efficacy. Oral cyclosporine is an effective systemic treatment in elderly AD, but can be used safely only for short time-periods (some weeks to few months) and long-term use of cyclosporine should be carefully considered due to the potential increased risks of toxicity on the cardiovascular system and kidney. About biological therapies, dupilumab blocks both IL-4 and IL-13, and markedly improves skin lesions and itch in adult as well as elderly patients with moderate-to-severe AD with a rapid response and non-serious adverse effects (2, 29–33). Dupilumab is also suitable for treating other Th2-mediated diseases, such as bronchial asthma and chronic rhinosinusitis with nasal polyposis (17, 27). Tralokinumab specifically blocks IL-13. Results from phase III clinical trials have demonstrated a substantial improvement in the severity and symptoms of AD after tralokinumab administration also in older adults (33), and real-life studies are beginning to be published (34, 35). Both dupilumab and tralokinumab are not generally immunosuppressive and do not require laboratory screening and monitoring, and are thus particularly indicated in elderly patients with AD (29–31). JAK proteins are involved in the proinflammatory cascade of several key cytokines, including those pathogenic in AD (2). The newer treatments for AD include several oral JAK inhibitors, including abrocitinib, baricitinib and upadacitinib. Abrocitinib is a JAK1 selective inhibitor and is licensed at a daily dose of 100 mg and 200 mg. Baricitinib is JAK1 and JAK2 inhibitor, approved at the dosage of 4 mg per day, with a reduction to 2 mg daily when possible. Upadacitinib is a selective and reversible JAK1 inhibitor, licensed at the dosage of 15 mg and 30 mg daily. In elderly patients with AD, the recommended dosage is 15 mg (2). Abrocitinib and upadacitinib have shown higher and/or faster efficacy compared with dupilumab in head-to-head trials (2, 29). Oral JAK inhibitors have a black box warning on increased risk for developing serious, life-threatening infections, such as active tuberculosis, invasive fungal infections, or infection with other opportunistic pathogens, and malignancies including lymphoma. The Food and Drug Administration (FDA) labels for tofacitinib, upadacitinib, and abrocitinib indicate a higher frequency of serious infections especially in older adults, the FDA label for baricitinib states that no overall differences in the safety and efficacy of the drug were observed between older and younger adults. FDA labels for abrocitinib and upadacitinib also indicate a higher frequency of malignancy in elderly patients, and an increased risk of major cardiovascular events, and thrombosis in patients with rheumatoid arthritis (36, 37). Based on data from the use of tofacitinib on rheumatoid arthritis, the European Medicines Agency (EMA) has recently raised concerns about the use of JAK inhibitors in patients older than 65 years and recommended caution in their use only when no suitable alternative treatment options are available (38). In the meantime, reports are accumulating indicating that JAK inhibitors may be a safe alternative in older adults (39).
A relevant issue with novel treatment for AD in older adults is the exclusion of these patients from clinical trials. Most of the trials limited the participation of older adults by either placing an upper age limit (ranging from age 40 to 70 years) or having eligibility criteria that would disproportionately exclude this population (40). In reviewing AD medications that are either newly approved or currently undergoing clinical trials, the proportion of older adults studied remains marginal. Despite being new therapies, on their labels both crisaborole and dupilumab state that clinical studies did not include a sufficient number of subjects 65 years or older to conclude whether the safety and efficacy of the drugs differed between older and younger adults. Older adults with AD are thus being treated with medications whose efficacy and safety were not explicitly validated among this age group.
CONCLUSION
AD is a heterogeneous disease from different perspectives (genetics, immunology, neurophysiology, clinics), which is also commonly observed in elderhood. In the elderly population, AD may have a high level of impact on quality of life and disease burden, frequently adding to an already complex comorbid situation. Thus, the proper recognition of AD in the elderly population is very important to avoid a high risk of underdiagnosis, but also to avoid overdiagnosis. In addition, understanding and full consideration of disease burden is crucial for choosing the most appropriate treatment. The mild-to-moderate forms are treated effectively with topical therapy, such as topical corticosteroids and topical calcineurin inhibitors. For the treatment of moderate-to-severe AD, there are many new systemic drugs available now and in the near future with distinct efficacy and safety profiles. Blocking IL-4/IL-13 pathways is effective in the short- and long-term, is well-tolerated and safe, and is not immunosuppressive. Moreover, there is no risk of deleterious drug interactions and no need for laboratory screening and monitoring. The efficacy of JAK inhibitors should be balanced with the possible side-effects.
REFERENCES
- Chiricozzi A, Maurelli M, Calabrese L, Peris K, Girolomoni G. Overview of atopic dermatitis in different ethnic groups. J Clin Med 2023; 12: 2701.
- Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema – part I: systemic therapy. J Eur Acad Dermatol Venereol 2022; 36: 1409–1431.
- Katsarou A, Armenaka M. Atopic dermatitis in older patients: particular points. J Eur Acad Dermatol Venereol 2011; 25: 12–18.
- Tanei R, Hasegawa Y. Atopic dermatitis in older adults: a viewpoint from geriatric dermatology. Geriatr Gerontol Int 2016; 16: 75–86.
- Girolomoni G, de Bruin-Weller M, Aoki V, Kabashima K, Deleuran M, Puig L, et al. Nomenclature and clinical phenotypes of atopic dermatitis. Ther Adv Chronic Dis 2021; 12: 20406223211002979.
- Chan LN, Magyari A, Ye M, Al-Alusi NA, Langan SM, Margolis D, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One 2021; 16: e0258219.
- Lusignan de S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. The epidemiology of eczema in children and adults in England: a population-based study using primary care data. Clin Exp Allergy 2021; 51: 471–482.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol 2013; 132: 1132–1138.
- Silverberg JI, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, Ong PY, et al. Distribution of atopic dermatitis lesions in United States adults. J Eur Acad Dermatol Venereol 2019; 33: 1341–1348.
- Williamson S, Merritt J, De Benedetto A. Atopic dermatitis in the elderly: a review of clinical and pathophysiological hallmarks. Br J Dermatol 2020; 182: 47–54.
- Bozek A, Fisher A, Filipowska B, Mazur B, Jarzab J. Clinical features and immunological markers of atopic dermatitis in elderly patients. Int Arch Allergy Immunol 2012; 157: 372–378.
- Yepes-Nuñez JJ, Guyatt GH, Gómez-Escobar LG, Pérez-Herrera LC, Chu AWL, Ceccaci R, et al. Allergen immunotherapy for atopic dermatitis: systematic review and meta-analysis of benefits and harms. J Allergy Clin Immunol 2023; 151: 147–158.
- Hui-Beckman JW, Goleva E, Berdyshev E, Leung DYM. Endotypes of atopic dermatitis and food allergy. J Allergy Clin Immunol 2023; 151: 26–28.
- Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines (Basel) 2021; 9: 303.
- Yosipovitch G, Gooderham MJ, Ständer S, Fonacier L, Szepietowski JC, Deleuran M, et al. Interpreting the relationship among itch, sleep, and work productivity in patients with moderate-to-severe atopic dermatitis: a post hoc analysis of JADE MONO-2. Am J Clin Dermatol 2023 Aug
25 . [Epub ahead of print]. - Bruin-Weller de M, Gadkari A, Auziere S, Simpson EL, Puig L, Barbarot S, et al. The patient-reported disease burden in adults with atopic dermatitis: a cross-sectional study in Europe and Canada. J Eur Acad Dermatol Venereol 2020; 34: 1026–1036.
- Beck LA, Cork MJ, Amagai M, De Benedetto A, Kabashima K, Hamilton JD, et al. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov 2022; 2:100131.
- Renert-Yuval Y, Del Duca E, Pavel AB, Fang M, Lefferdink R, Wu J, et al. The molecular features of normal and atopic dermatitis skin in infants, children, adolescents, and adults. J Allergy Clin Immunol 2021; 148: 148–163.
- Del Duca E, Renert-Yuval Y, Pavel AB, Mikhaylov D, Wu J, Lefferdink R, et al. Proteomic characterization of atopic dermatitis blood from infancy to adulthood. J Am Acad Dermatol 2023; 88: 1083–1093.
- Zhou L, Leonard A, Pavel AB, Malik K, Raja A, Glickman J, et al. Age-specific changes in the molecular phenotype of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2019; 144: 144–156.
- Wang S, Zhu R, Gu C, Zou Y, Yin H, Xu J, et al. Distinct clinical features and serum cytokine pattern of elderly atopic dermatitis in China. J Eur Acad Dermatol Venereol 2020; 34: 2346–2352.
- Facheris P, Da Rosa JC, Pagan AD, Angelov M, Del Duca E, Rabinowitz G, et al. Age of onset defines two distinct profiles of atopic dermatitis in adults. Allergy 2023; 78: 2202–2214.
- Liu P, Zhao Y, Mu ZL, Lu QJ, Zhang L, Yao X, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin Med J (Engl) 2016; 129: 757–762.
- Yue W, Cheng D, Sun Z, Shen Y, Wang S, Liu X, et al. Validation of diagnostic criteria for atopic dermatitis and proposal of novel diagnostic criteria for adult and elderly Chinese populations: a multicentre, prospective, clinical setting-based study. Br J Dermatol 2023; 188: 420–426.
- Saeki H, Ohya Y, Furuta J, Arakawa H, Ichiyama S, Katsunuma T, et al. Committee for clinical practice guidelines for the management of atopic dermatitis 2021, the Japanese society of allergology, The Japanese Dermatology Association. Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int 2022; 71: 448–458.
- Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema - part II: non-systemic treatments and treatment recommendations for special patient populations. J Eur Acad Dermatol Venereol 2022; 36: 1904–1926.
- Kolkhir P, Akdis CA, Akdis M, Bachert C, Bieber T, Canonica GW, et al. Type 2 chronic inflammatory diseases: targets, therapies and unmet needs. Nat Rev Drug Discov 2023; 22: 743–767.
- Dhadwal G, Albrecht L, Gniadecki R, Poulin Y, Yeung J, Hong CH, et al. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section IV: treatment options for the management of atopic dermatitis. J Cutan Med Surg 2018; 22: 21S–29S.
- Adam DN, Gooderham MJ, Beecker JR, Hong CH, Jack CS, Jain V, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol Venereol 2023; 37: 1135–1148.
- Patruno C, Napolitano M, Argenziano G, Peris K, Ortoncelli M, Girolomoni G, et al. Dupilumab therapy of atopic dermatitis of the elderly: a multicentre, real-life study. J Eur Acad Dermatol Venereol 2021; 35: 958–964.
- Russo F, Milanesi N, Cartocci A, Bruzziches F, Tronconi G, Lazzeri L, et al. Dupilumab in elderly patients with severe atopic dermatitis. Dermatitis 2021; 32: S24–S27.
- Silverberg JI, Lynde CW, Abuabara K, Patruno C, de Benedetto A, Zhang H, et al. Efficacy and safety of dupilumab maintained in adults ≥ 60 years of age with moderate-to-severe atopic dermatitis: analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol 2023; 24: 469–483.
- Merola JF, Butler DC, Mark T, Schneider S, Kim Y, Abuabara K. Safety and efficacy of tralokinumab in older adults with moderate-to-severe atopic dermatitis: a secondary analysis. JAMA Dermatol JAMA Dermatol 2023; 159: 1119–1123.
- Pezzolo E, Schena D, Gambardella A, Rossi M, Barei F, Calzavara Pinton P, et al. Survival, efficacy and safety of tralokinumab after 32 and 52 weeks of treatment for moderate-to-severe atopic dermatitis in adults: a multicentre real-world study. J Eur Acad Dermatol Venereol 2023 Jul
22 . [Epub ahead of print]. - García Castro R, Heras Mendaza F, Santiago Sánchez-Mateos DI, Fariña Sabrís MC, Alcaraz León I. First short-term effectiveness and security data of tralokinumab in severe atopic dermatitis in real clinical practice. Dermatitis 2023 May
2 . [Epub ahead of print]. - Philippoteaux C, Deprez V, Nottez A, Cailliau E, Houvenagel E, Deprez X, et al. Characteristics of patients treated with JAK inhibitors in rheumatoid arthritis before versus after VTE risk warnings. J Clin Med 2022; 12: 207.
- Winthrop KL, Cohen SB. Oral surveillance and JAK inhibitor safety: the theory of relativity. Nat Rev Rheumatol 2022; 18: 301–304.
- https://www.ema.europa.eu/en/news/ema-recommends-measures-minimise-risk-serious-side-effects-janus-kinase-inhibitors-chronic. [accessed 5 May 2023]
- Piscazzi F, Gargiulo L, Ibba L, Valenti M, Facheris P, Costanzo A, et al. Upadacitinib for the treatment of atopic dermatitis in the elderly: an Italian case series of seven patients. J Dermatolog Treat 2023; 34: 2245510.
- Lam M, Zhu JW, Maqbool T, Adam G, Tadrous M, Rochon P, et al. Inclusion of older adults in randomized clinical trials for systemic medications for atopic dermatitis: a systematic review. JAMA Dermatol 2020; 156: 1240–1245.