SHORT COMMUNICATION
Botanical Extract Coacillium for Management of Paediatric Atopic Dermatitis: A Case Report
1Centre Dermatologique et Dermato-chirurgical des Croisettes/Groupe Vidymed, Route de la Corniche 1, CH-1066 Epalinges, Vaud, Switzerland and 2Department of Dermatology, Central University Hospital Vaudois, Lausanne, Vaud, Switzerland. E-mail: Philipp.spring@vidymed.ch
Citation: Acta Derm Venereol 2023; 103: adv13376. DOI: https://doi.org/10.2340/actadv.v103.13376.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Oct 18, 2023; Published: Nov 21, 2023
INTRODUCTION
Atopic dermatitis (AD) is associated with the activation of type 2 inflammation (1). Environmental and psychological factors complete the aetiological characteristics and aggravate the clinical picture (2). In addition to recently highlighted therapeutic approaches (anti-IL13, anti-PD4, anti-JAK) (3), anti-inflammatory herbal mixtures have been studied (4). Transient receptor potential cation channel subfamily M member 8 (TRPM8) and cannabinoid agonists represent novel pathways, but also Coacillium (Coa) acting as an immunomodulator in the type 2 inflammatory pathway but also through pro-apoptotic B Cell Lymphoma 2-6 gene expression (BCL2-6) (5). Coa is a topical hydroalcoholic solution composed of 4 plants extracts: Onion (Allium cepa), Lemon (Citrus limon), Guarana (Paullinia cupana) and Theobroma cacao. Coa was initially commercialized as an anti-hair loss lotion and is now being studied as a drug for treatment of alopecia areata in children (6). Coacillium is the only name used for the product. It has not yet been approved for any indication in Switzerland but has been filed for approval with the European Medical Agency (EMA) for the treatment of Alopecia Areata as a « Herbal Medicine ». We report here the experimental use of Coa to manage symptoms in a paediatric patient with AD.
MATERIALS AND METHODS
The product used in this case report is an alcohol-free Coa formulation (with reliable test data related to safety)(7). The constituent plant extracts listed in the introduction are all classed as Generally Regarded as Safe (GRAS) or equivalent by the American Food and Drug Administration (FDA), who placed the development and regulation of Coa under the category of “Botanical Drugs” (also called “herbal medicine” by EMA). Unlike chemical entities, botanical drugs do not have International Nonproprietary Names (INN). The alcohol-free product batch 18 3 21 5, manufactured by Delpharm Tours, Chambray-Les-Tours, France, contains the following excipients regularly used in cutaneous solutions: water, glycerin, sodium chloride, potassium sorbate, sodium benzoate, maltodextrin, citric acid. Coa has no allergenic potential. In this case, Coa was used as an adjunctive therapy to decrease the sensation of pruritus and to control skin lesions in a patient with AD. An 8-year-old girl presented with persistent cheilitis with intermittent acute flares. Dry skin, erythema, desquamation, and the Dennie-Morgan sign associated with nocturnal pruritus complete the clinical picture of diagnosed AD in early childhood (Fig. 1A). Previous conventional approaches such as topical corticosteroids led to a partially favourable situation.
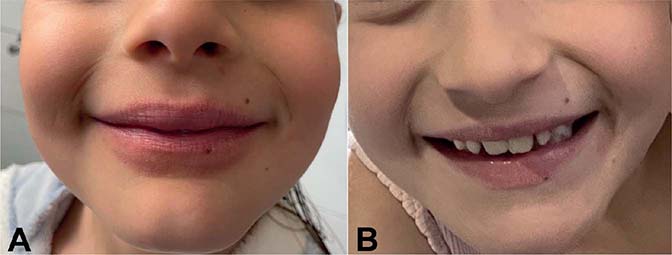
Fig. 1. Atopic cheilitis (A) before treatment and (B) after 1 month of treatment.
RESULTS
The patient experienced immediate improvement with a significant decrease in itch, dryness, rhagades, and inflammation with 1 application locally (fingertip-unit amount) daily for 5 days, which allowed us to taper the treatment (1-month duration interval therapy twice a week). Usual prior to Coa moisturizing treatment (products containing a combination of humectants, emollients and occlusives for general skincare) and, especially for the lips, a protective barrier function with ingredients such as shea butter and cocoa butter with very low allergenic potential allowed long-term control of her disease (Fig. 1B). To date, in the 12 months after treatment, she has not experienced major worsening of her symptoms.
DISCUSSION
The patient in this single case report experienced subjective improvement of her AD symptoms: an immediate anti-inflammatory response with long-term maintenance of symptom control. Long-term use of corticosteroids, even at regular intervals, can often pose problems with compliance and side-effects (vasoconstriction, fragility of the skin barrier). Calcineurin inhibitors are often poorly tolerated due to the associated mast cell stimulation, especially on the lips. Many patients with AD or their parents seek alternatives to standard treatment. This single preliminary case study indicates the potential of an herbal medicine in the management of AD. No contact sensitization due to Coa has yet been shown. There are a few reports of allergic reactions following onion (Allium cepa) consumption (8). The CoA path mechanism, mainly around the B Cell Lymphoma gene 2 (BCL-2) expression affinity, suggests the involvement of apoptosis in AD (1,5). This intrinsic process (mitochondrial driven pathway), where apoptosis is driven by cytotoxic drugs or DNA-damage, is regulated by BCL-2 family proteins and the apoptosis-inducing factor (9). This single case report found Coa to be a possible therapeutic modality. However, further research is needed to determine its efficacy.
ACKNOWLEDGEMENTS
Funding sources: Legacy Healthcare Ltd, Route de la Corniche 3B, 1066 Epalinges, Vaud, Switzerland.
Conflicts of interest: The author works as a consultant for Legacy Healthcare Ltd. He receives honoraria related to the academic activity of the company.
REFERENCES
- Braun C, Vocanson M, Nicolas JF, Nosbaum A. Physiopathologie de la dermatite atopique et des autres maladies atopiques: une approche globale est-elle possible? Ann Dermatol Venereol 2020; 147: 11S4–11S11.
- Eichenfield LF, Kusari A, Han AM, Barbarot S, Deleuran M, Lio P, et al. Therapeutic education in atopic dermatitis: a position paper from the International Eczema Council. JAAD Int 2021; 5: 8–13.
- Newsom M, Bashyam AM, Balogh EA, Feldman SR, Strowd LC. New and emerging systemic treatments for atopic dermatitis. Drugs 2020; 80: 1041–1052.
- Yan F, Li F, Liu J, Ye S, Zhang Y, Jia J, et al. The formulae and biologically active ingredients of Chinese herbal medicines for the treatment of atopic dermatitis. Biomed Pharmacother 2020; 127: 110142.
- Katoulis AC, Liakou A, Alevizou A, Bonovas S, Bozi E, Kontogiorgi D, et al. Efficacy and safety of a topical botanical in female androgenic alopecia: a randomized single-blinded vehicle-controlled study. Skin Appendage Disord 2018; 4: 160–165.
- Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol 2018; 78: 15–24.
- Blume-Peytavi U, Piraccini BM, Reygagne P, Mukherjee B, Guichard A, Pralong W, et al. Efficacy and safety of Coacillium in children and adolescents with moderate to severe alopecia areata: a randomised, double-blind, multicentre, phase 2-3 trial. EADV 2023; Abstract Number: 1907.
- Albanesi M, Pasculi C, Giliberti L, Rossi MP, Di Bona D, Caiafa MF, et al. Immunological characterization of onion (Allium cepa) allergy. Postepy Dermatol Alergol 2019; 36: 98–103.
- Szymansky L, Cios A, Ciepielak M, Stankiewicz W. Cytokines and apoptosis in atopic dermatitis. Postepy Dermatol Alergol 2021; 38: 1–13.