ORIGINAL REPORT
Differential Immunoexpression of Inhibitory Immune Checkpoint Molecules and Clinicopathological Correlates in Keratoacanthoma, Primary Cutaneous Squamous Cell Carcinoma and Metastases
Anke S. LONSDORF1, Dominic EDELMANN2,3, Thomas ALBRECHT4, Alexander BROBEIL4,5, Jannik LABRENZ2,3, Moritz JOHANNING2,3, Richard F. SCHLENK2,3, Benjamin GOEPPERT4,6 Alexander H. ENK1 and Ferdinand TOBERER1
1Department of Dermatology, University Hospital Heidelberg, 2German Cancer Research Center, 3NCT Trial Center, National Center for Tumor Diseases, German Cancer Research Center and Heidelberg University Hospital, 4Institute of Pathology, Heidelberg University Hospital, Heidelberg and 5Tissue Bank of the National Center for Tumor Diseases, Heidelberg, and 6Institute of Pathology and Neuropathology, RKH Klinikum Ludwigsburg, Germany
Beyond established anti-programmed cell death protein 1/programmed cell death ligand 1 immunotherapy, T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) and its ligand CD155 are promising novel inhibitory immune checkpoint targets in human malignancies. Yet, in cutaneous squamous cell carcinoma, evidence on the collective expression patterns of these inhibitory immune checkpoints is scarce. Complete tumour sections of 36 cutaneous squamous cell carcinoma, 5 cutaneous metastases and 9 keratoacanthomas, a highly-differentiated, squamoproliferative tumour, with disparately benign biologic behaviour, were evaluated by immunohistochemistry for expression of programmed cell death ligand 1 (Tumor Proportion Score, Immune Cell Score), TIGIT, CD155 and CD8+ immune infiltrates. Unlike keratoacanthomas, cutaneous squamous cell carcinoma displayed a strong positive correlation of programmed cell death ligand 1 Tumor Proportion Score and CD115 expression (p < 0.001) with significantly higher programmed cell death ligand 1 Tumor Proportion Score (p < 0.001) and CD155 expression (p < 0.01) in poorly differentiated G3-cutaneous squamous cell carcinoma compared with keratoacan-thomas. TIGIT+ infiltrates were significantly increased in programmed cell death ligand 1 Immune Cell Score positive primary tumours (p = 0.05). Yet, a strong positive correlation of TIGIT expression with CD8+ infiltrates was only detected in cutaneous squamous cell carcinoma (p < 0.01), but not keratoacanthomas. Providing a comprehensive overview on the collective landscape of inhibitory immune checkpoint expression, this study reveals associations of novel inhibitory immune checkpoint with CD8+ immune infiltrates and tumour differentiation and highlights the TIGIT/CD155 axis as a potential new target for cutaneous squamous cell carcinoma immunotherapy.
Key words: checkpoint inhibition; PD-L1; TIGIT; CD155; immune evasion; keratoacanthoma; squamous cell carcinoma.
SIGNIFICANCE
In some patients with advanced cutaneous squamous cell carcinoma, a common skin cancer, immunotherapy targeting programmed cell death protein 1/programmed cell death ligand 1 shows limited efficacy, which may potentially be improved by targeting other inhibitory immune checkpoints. Assessing cutaneous squamous cell carcinoma, metastases and keratoacanthoma, a skin tumour with rather benign behaviour, the current study found associations of programmed cell death ligand 1 expression patterns and the novel inhibitory immune checkpoints T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain/CD155, with low tumour differentiation, a negative prognosticator, and infiltrating immune cells. The current results suggest a potential value of immunotherapy via programmed cell death ligand 1 and T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain/CD155 inhibition for advanced cutaneous squamous cell carcinoma.
Citation: Acta Derm Venereol 2024; 104: adv13381. DOI https://doi.org/10.2340/actadv.v104.13381.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: May 23, 2023; Accepted: Dec 20, 2023; Published: Feb 7, 2024
Corr: Anke Lonsdorf, Department of Dermatology, University Hospital Heidelberg, Im Neuenheimer Feld 440, 69120 Heidelberg, Germany. E-mail: anke.lonsdorf@med.uni-heidelberg.de
Competing interests and funding: AEH receives funding by grant SFB/TR 156 of the DFG (Deutsche Forschungsgemeinschaft) and payment or honoraria by Janssen-Cilag, Biotest, MSD and Galderma. AEH is president of the European Dermatology Forum (EDF). DE received lecture fees from Pfizer. All other authors have no conflicts of interest to declare.
INTRODUCTION
Accounting for approximately 20% of all cutaneous malignancies, cutaneous squamous cell carcinoma (cSCC) is the second most common type of keratinocyte cancer (KC), also referred to as non-melanoma skin cancer (1), the overall most prevalent malignancy in fair-skinned populations (2, 3). While most primary cSCC may be cured by surgical excision, locally advanced and metastatic cSCC is associated with substantial morbidity, mortality and a significant impact on quality of life and healthcare burden (4–6).
Tumour-mediated immunosuppression, achieved i.a. by expression of surface molecules, that hamper anti-tumour effector functions of tumour infiltrating leucocytes (TIL) in the tumour microenvironment (TME), is common in cSCC (5, 7). Targeted activation or interruption of inhibitory immune checkpoints (iICP) by therapeutic antibodies in order to improve anti-cancer immune responses evolved as an effective therapeutic approach for multiple advanced cancers. (4, 8–10). Particularly monoclonal antibodies targeting the programmed cell death protein 1/programmed cell death ligand 1 (PD1-/PD-L1) axis, an iICP, maintaining peripheral tolerance and impeding autoimmunity, demonstrated robust and durable anti-tumour activity and emerged as new standard of care for advanced cSCC (4, 9, 11). However, PD-L1 immunoexpression is not regarded a predictive biomarker for response to anti-PD-1/PD-L1 therapy in cSCC (9, 12–14) and in subsets of patients the therapeutic benefit from available iICP inhibitors is limited by therapy resistance (12, 15), indicating the need for the examination of novel iICP and, conceivably, combinational therapeutic strategies for advanced cSCC (15).
The T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif (ITIM) domain (TIGIT), a member of the immunoglobulin super family, has recently been implicated in regulating CD8+ T cell effector functions independent of PD-1/PD-L1 engagement (16, 17). As an inhibitory counterpart of the co-stimulatory receptor CD226, the surface molecule TIGIT binds with higher affinity to CD155 (poliovirus receptor, PVR), their mutual ligand, thus opposing CD226 co-stimulation (16–18). In melanoma, CD155 has been recognized as an additional iICP impairing anti-tumour T cell and NK cell immunity and promoting tumour invasiveness (19).
We recently reported on PD-L1 as negative prognosticator and the potential relevance of the TIGIT/CD155 axis in Western-world gallbladder cancer (20). However, current knowledge co-expression of these iICP in cSCC and its TME is scarce. Here, we comprehensively assessed the collective landscape of immunoexpression patterns of PD-L1, TIGIT and CD155 along with CD8+ TIL in primary cSCC and cutaneous metastases. We further examined the reciprocal correlation of these iICPS across all differentiation stages of primary cSCC, including analyses of keratoacanthoma (KA), a highly-differentiated, squamoproliferative, epithelial tumour, with disparately benign biologic behaviour through spontaneous regression and extremely low potential for metastasis (21, 22), and disclose potential associations with clinically relevant parameters.
MATERIALS AND METHODS
Patients and tumours
Tissue samples from 50 patients were analysed, comprising 9 KA, 36 cSCC and 5 cutaneous metastases. All patients underwent routine surgery for their tumours at the Department of Dermatology, University Hospitals of Heidelberg, Germany. Notably, no patient had received prior immunotherapy or iatrogenic immunosuppression. Diagnosis and histopathological features of all samples were determined by board-certified dermatopathologists. Formalin-fixed paraffin embedded (FFPE) samples were retrieved from the tissue bank of the Department of Dermatology, University Hospitals Heidelberg (Biobank of the National Center for Tumor Diseases Heidelberg, Germany) in accordance with institutional guidelines. The study was approved by the ethics committee of Heidelberg University and all participants gave prior written informed consent.
Immunohistochemistry
Immunohistochemistry (IHC) of serial 3-μm FFPE tissue sections was performed at the Biobank of the NCT Heidelberg, largely as described previously (20), on an automated immunostainer (Ventana BenchMark Ultra, Ventana Medical Systems, Tuscon, USA) with monoclonal antibodies against PD-L1 (clone SP263, Roche, Rotkreuz, Switzerland) and CD8 (clone SP57, Roche) at the provided dilutions of the ready-to-use kits. CD155 IHC was performed with a monoclonal anti-CD155 antibody (clone D8A5G, 1:200, Cell Signaling Technology, Danvers, MA, USA) and visualization by the biotin-free OptiView DAB IHC Detection Kit (Ventana MedicalSystems, Oro Valley, AZ, USA). TIGIT IHC was performed with a monoclonal anti-TIGIT antibody (clone TG1, 1:100, OncoDianova, Germany) on another automated immunostainer (Autostainer Link 48, Agilent Technologies Inc., Santa Clara, CA, USA) using AEC-Chromogen (Dako REAL EnVision Detection System, Dako, Agilent Technologies Inc., Santa Clara, USA) and antigen retrieval in citrate buffer (pH 6.0). Germinal centres of normal human tonsil tissue, showing high TIGIT expression (23), served as positive control for TIGIT IHC. Slides were counterstained with hemalaun.
Evaluation of immunostaining
Entire tissue slides were evaluated to eliminate confounding effects due to intratumoural heterogeneity, which has been reported for iICP expression, particularly PD-L1 (24, 25). Evaluation of immunostaining was performed by a board certified dermatopathologist and reproducibility was verified by evaluation of randomly selected samples by an additional pathologist, both blinded to clinical parameters. PD-L1 staining of the epithelial tumour component and of TIL in the peritumoural stroma were analysed separately and expressed as PD-L1 Tumor Proportion Score (TPS), defined as the percentage of viable tumour cells exhibiting complete or partial membranous staining at any intensity above background cytoplasmic staining, and PD-L1 Immune Cell Score (IC), respectively, as previously defined (26). In line with current consensus, only membranous PD-L1 staining was considered positive for the epithelial tumour component, while for TIL both cytoplasmatic and membranous staining were regarded as positive. Based on recent references, PD-L1 TPS positivity was defined as ≥ 1% stained tumour cells (27–30). CD8 and TIGIT immunostainings were regarded as positive in case of specific cytoplasmic or membranous staining and expressed as the proportion of positive immune cells relative to the tumour cell area. CD155 immunostaining was considered positive only in case of a specific membranous staining and expressed as percentage of analysed cells, as previously described (20). Cut-off-values for TIGIT and CD155 positivity in cSCC are not or insufficiently established and were defined at ≥ 5% and ≥ 1%, respectively, for statistical evaluation as previously proposed (20, 31, 32), while the median value was used as cut-off to discriminate high and low density of CD8+ immune infiltrates.
Statistical analysis
All statistical analyses were performed with R version 4.2.2 (33). Differences in clinicopathological characteristics between groups defined by immunoexpression status were assessed with Fisher’s exact test or Student’s t-test (for age). Differences in quantitative (non-dichotomized) expression between subgroups were assessed with Wilcoxon’s signed-rank test. Spearman’s rank correlation and a corresponding test based on algorithm AS89 was used for the association of 2 quantitative expression measures. p ≤ 0.05 was considered statistically significant. Since all analyses were exploratory, no adjustments for multiple comparisons were performed.
RESULTS
Clinicopathological characteristics of patients and tumours are summarized in Table I. Exploratory analyses for tumour tissues from 50 patients, predominantly men (n = 39, 78.0%) at a median age of 78 years (range 48–95 years) were conducted. The vast majority of tumours, comprising 36 primary cSCC at diverse differentiation stages (G1–G3), 9 KA and 5 cutaneous metastases, was localized in chronic sun-exposed body sites, mostly the head and neck region (n = 35, 70.0%).
Immunohistochemical staining
Representative images of cSCC stained for PD-L1, TIGIT, CD155 and CD8+ TIL are shown in Fig. 1. PD-L1 immunoexpression on tumour cells and immune cells was evaluated in all samples. TIGIT and CD8 immunostaining was successfully evaluated in 49 tissue specimens. One specimen had to be excluded due to insufficient tumour material that did not meet the evaluation criteria (drop-out rate 2%). TIGIT expression was found to be exclusively expressed by mononuclear immune cells with absent immunoreactivity in cSCC tumour epithelium. By contrast, CD155 was exclusively expressed by tumour cells with foremost membranous staining. CD8+ immune cell infiltrates were detected in all samples.
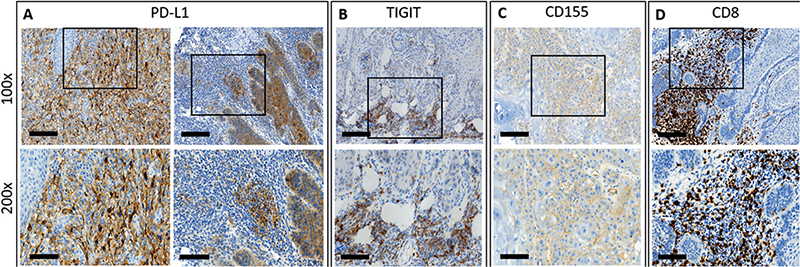
Fig. 1. Immunohistochemical staining. Representative examples of programmed death-ligand 1 (PD-L1) expression of tumour cells (A, left panels; PD-L1 Tumor Proportion Score (TPS) of whole tumour section: 90%) and immune cell infiltrates (A, right panels; PD-L1 Immune Cell Score (IC) of whole tumour section: 20%), T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) (B; TIGIT expression of whole tumour section: 40%) and CD155 immunoexpression (C; CD155 expression of whole tumour section: 50%) and CD8+ infiltrating immune cells (D; CD8+ tumour infiltrating leucocytes (TIL) density of whole tumour section: 50%) in cutaneous squamous cell carcinoma. Selected tissue slides were digitalized using a NanoZoomer S60 slide scanner (Hamamatsu Photonics Deutschland GmbH, Herrsching am Ammersee, Germany) for image acquisition with Aperio Image Scope Software (version 12.4.0.5043, Leica Biosystems, Nussloch, Germany). Original magnification ×100 (upper panels; scale bars: 200 µm) and insert ×200 (lower panels; scale bars: 100 µm).
Correlations of iICP positivity and CD8+ profiles with clinicopathological parameters
To determine whether specific clinicopathological criteria might predict iICP profiles, available clinicopathological parameters of the entire cohort were stratified for positivity for PD-L1, TIGIT, CD155 and CD8+ TIL density at indicated cut-offs (Table II). While PD-L1 TPS positivity was significantly more likely with increasing age, sex had no impact on positivity for PD-L1, TIGIT, CD155 or density of CD8+ TIL infiltration. Tumour thickness ≥ 6 mm, an established risk factor for both tumour recurrence and metastasis (4, 5), showed no significant overall association with iICP expression or CD8+ TIL density. Yet, the majority of primary tumours thicker than ≥ 6 mm displayed TIGIT positivity (19/20, 95.0%) and tumours with TIGIT expression below cut-off were mostly thinner (7/8, 87.5%). Tumour type (p < 0.01) and differentiation (p < 0.01) were significantly associated with PD-L1 TPS positivity, with a higher proportion of PD-L1 TPS positive primary cSCC (24/36, 66.7%) compared with KA (1/9, 11.1%) or metastases (1/5, 20.0%). The majority of G3-cSCC displayed PD-L1 TPS positivity (13/16, 81.3%), in sharp contrast to highly differentiated KA (1/9, 11.1%). Furthermore, all KA (9/9, 100%) and the vast majority of G3-cSCC (14/16, 87.5%) displayed a positive TIGIT expression status. In addition, tumour differentiation was significantly associated with CD8+ TIL density (p < 0.05) with the majority of KA displaying high CD8+ density (6/9, 66.7%) and typically low CD8+ TIL infiltration in G1-cSCC (8/8, 100%) and G3-cSCC (10/16, 62.5%). While CD155 positivity was particularly common in G3-cSCC (13/16, 81.3%), no significant overall association with clinicopathological parameters was noted.
iICP immunoexpression and CD8+ immune infiltrates according to differentiation of primary tumours and of cutaneous metastases
Since low differentiation of cSCC is an established risk factor for both tumour recurrence and metastasis (4, 5), this study analysed PD-L1, TIGIT and CD155 expression and CD8+ TIL infiltration individually in well-differentiated (G1, n = 9), moderately-differentiated (G2, n = 11) and poorly-differentiated primary cSCC (G3, n = 16) as well as in highly differentiated KA (n = 9) and cutaneous metastasis (n = 5) (Fig. 2). A significantly higher PD-L1 TPS was detected in G3-cSCC compared with G1-cSCC (p < 0.05), G2-cSCC (p < 0.05), KA (p < 0.001) and metastases (p < 0.05) (Fig. 2A), while no significant differences in PD-L1 IC were noticed across specimens (Fig. 2B). The proportion of TIGIT+ cells was significantly lower in G3-cSCC (p < 0.001) and metastasis (p < 0.05) compared with highly differentiated KA while differences among G1-, G2- and G3-cSCC or metastasis were not statistically significant (Fig. 2C). Contrasting the high portion of TIGIT+ infiltrates in KA and low proportion of TIGIT+ infiltrates seen in G3-cSCC, the TIGIT ligand CD155 exhibited a reverse expression pattern (Fig. 2 D). The overall lowest proportion of CD155+ tumour cells was found in highly differentiated KA with a significantly higher proportion of CD155+ tumour cells in G3-cSCC (Fig. 2D, p < 0.01). Differences in CD155 expression among G1- and G2-cSCC compared with G3-cSCC and metastasis were not statistically significant (Fig. 2D). A significantly higher proportion of CD8+ TIL was noted in KA compared with G1-cSCC (Fig. 2E, p < 0.01), which displayed the overall lowest CD8+ infiltration. The second lowest CD8+ TIL density was found in metastases (Fig. 2E).
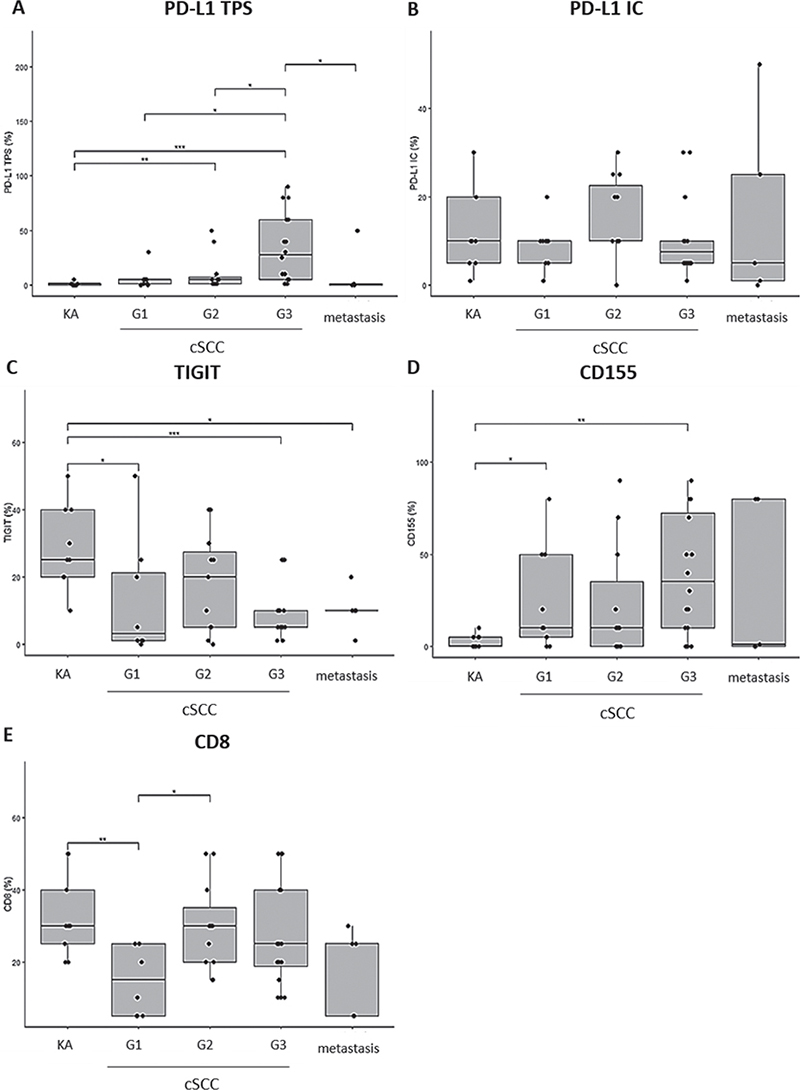
Fig. 2. Programmed death-ligand 1 (PD-L1), T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) and CD155 expression and CD8+ immune infiltrates in keratoacanthoma across different degrees of differentiation (G1–G3) of primary cutaneous squamous cell carcinoma (cSCC) and cutaneous metastases. (A) PD-L1 immunoexpression (Tumor Proportion Score (TPS), %). (B) PD-L1 immunoexpression (Immune Cell Score (IC), %). (C) TIGIT immunoexpression (%). (D) CD155 immunoexpression (%). (E) CD8 immunoexpression (%). p-values are obtained by applying Wilcoxon’s ranked-sum test. p<0.05 was considered statistically significant. *p < 0.05; **p < 0.01; ***p < 0.001. G1: well differentiated; G2: moderately differentiated; G3: poorly differentiated; KA: keratoacanthoma.
Reciprocal relationships between iICP expression patterns and CD8+ host immunity
To study the reciprocal relationships between iICP expression patterns and CD8+ host immunity in the TME this study stratified primary tumours (i.e. cSCC and KA) for positive iICP expression at indicated cut-offs and the density of CD8+ TIL (Fig. 3). While stratification for PD-L1 and CD155 positivity did not show significant differences regarding CD8+ TIL densities (Fig. 3A and D), TIGIT positive primary tumours showed significantly increased CD8+ TIL compared with TIGIT negative primary tumours (Fig. 3C, p < 0.05). Furthermore, TIGIT+ infiltrates were significantly increased in PD-L1 IC-positive tumours (Fig. 3F, p = 0.05), while stratification for tumoural PD-L1 expression did not reveal a significant difference (Fig. 3E, p = 0.13). Conversely, PD-L1 TPS-positive specimens displayed significantly higher tumoural expression of the TIGIT ligand CD155 (Fig. 3G, p < 0.001), while the difference in CD155 expression did not reach significance when tumours were stratified for PD-L1 IC positivity (Fig. 3H) or TIGIT (Fig. 3I).
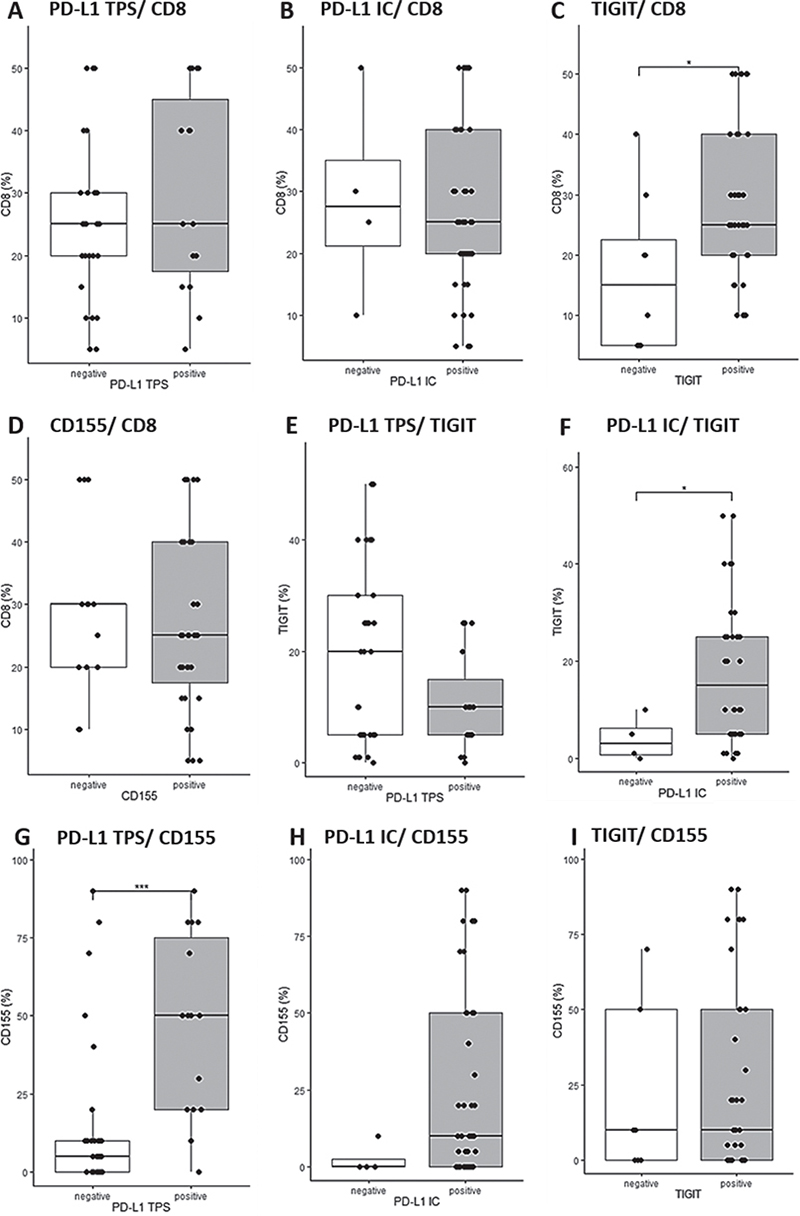
Fig. 3. Reciprocal relationships between inhibitory immune checkpoint (iICP) expression patterns and CD8+ host immunity in cutaneous squamous cell carcinoma and keratoacanthoma. (A) Programmed death-ligand 1 (PD-L1) expression status (Tumor Proportion Score (TPS))/CD8 immunoexpression (%). (B) PD-L1 expression status (Immune Cell Score (IC))/CD8 immunoexpression (%). (C) T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) expression status/CD8 immunoexpression (%). (D) CD155 expression status/CD8 immunoexpression (%). (E) PD-L1 expression status (TPS)/TIGIT immunoexpression (%). (F) PD-L1 expression status (IC)/TIGIT immunoexpression (%). (G) PD-L1 expression status (TPS) /CD155 immunoexpression (%). (H) PD-L1 expression status (IC)/CD155 immunoexpression (%). (I) TIGIT expression status/CD155 immunoexpression (%). Cut-off values: PD-L1 TPS: negative: <1%, positive: ≥1%; PD-L1 IC: negative: < 5%, positive ≥ 5%; TIGIT: negative: < 5%, positive ≥ 5%; CD155: negative: < 1%, positive: ≥ 1%.* p < 0.05; ***p < 0.001; p - values are obtained by applying Wilcoxon’s ranked-sum test. p<0.05 was considered statistically significant.
Correlation of iICP co-expression patterns and CD8+ infiltrates
To gain additional insights into co-expression patterns, this study examined the correlations among iICP expression as well as CD8+ TIL densities across diverse grades of histopathological differentiation of primary tumours (Fig. 4). Correlations between PD-L1 TPS or IC and CD8+ TIL were not statistically significant in cSCC or KA (Fig. 4A and B). A strong positive correlation of TIGIT expression with CD8+ infiltrates was detected in cSCC (Fig. 4C, p < 0.01, r = 0.52), whereas an inverse (but non-significant) correlation was found in KA (Fig. 4C, p = 0.25, r = –0.43). Considering CD155 expression, no significant correlations with CD8+ infiltrates were found (Fig. 4D, cSCC: p = 0.32, r = 0.17; KA: p = 0.36, r = –0.34). Furthermore, no significant correlations between TIGIT+- infiltrates and PD-L1 TPS (Fig. 4E, cSCC: p = 0.89, r = –0.025; KA: p = 0.88, r = –0.06) nor PD-L IC (Fig. 4F, cSCC: p = 0.1, r = –0.28; KA: p = 0.18, r = –0.65) were noted. By contrast, tumoural CD155 expression correlated positively with tumoural PD-L1 expression solely in cSCC (Fig. 4G, p < 0.001, r = 0.56), For CD155 and PD-L1 IC no significant correlations were noted (Fig. 4H, cSCC: p = 0.27, r = 0.19, KA: p = 0.62, r = –0.19), whereas a positive correlation for CD155 and TIGIT expression was found in KA (Fig. 4I, p < 0.05, r = 0.79), but not cSCC (Fig. 4I, p = 0.12, r = 0.27).
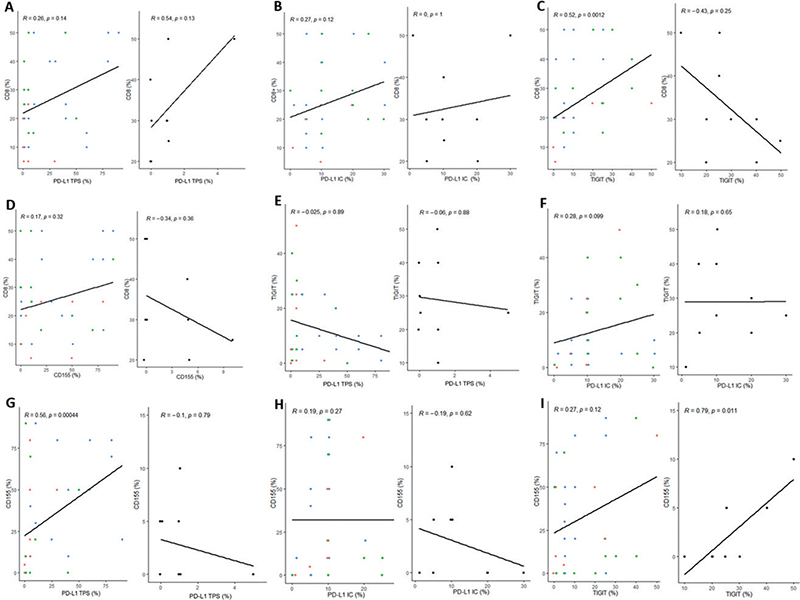
Fig. 4. Correlation of inhibitory immune checkpoint (iICP) co-expression patterns and CD8+ infiltrates in cutaneous squamous cell carcinoma and keratoacanthoma. (A–I) left panel: cutaneous squamous cell carcinoma (tumour differentiation: red: G1, green: G1, blue: G3); (A–I) right panel: keratoacanthoma (black). Spearman’s rank correlation was used. p-values for Spearman’s test were calculated using Algorithm AS89. p < 0.05 was considered statistically significant. IC: Immune Cell Score; PD-L1: programmed death-ligand 1; TIGIT: T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain; TPS: Tumor Proportion Score; G1: well differentiated; G2: moderately differentiated; G3: poorly differentiated.
DISCUSSION
Antibodies targeting iICP, such as the PD-1/PD-L1 axis, have broadened the therapeutic armamentarium available for patients with advanced cancers, including cSCC. Although PD-L1 expression is the most widely adopted parameter for selection of patients that may profit from immunotherapy (34), PD-L1 expression is only modestly correlated with clinical outcome in cSCC and other cancers (12, 14). Furthermore, for non-responders to anti- PD-1/PD-L1 immunotherapy, established therapeutic options beyond conventional chemotherapy or EGFR-inhibitors are vastly lacking for advanced cSCC (7, 11). Emerging evidence acknowledges the TIGIT/CD155 axis as a promising novel iICP in human malignancies (16, 17, 32) and suggests that combinational targeting of PD-1/PD-L1 and TIGIT/CD155, might restore CD8+ TIL function and improve anticancer control (35, 36).
While the value of PD-L1 as a prognostic marker in cSCC remains controversial, PD-L1 expression has been associated with high metastatic risk and poor prognosis in several studies (28, 37). In line with previous research (28, 38), differentiation of primary tumours, an established prognosticator for metastatic risk, had a significant impact on tumoural PD-L1 expression in the current study, with a particularly high proportion of PD-L1 TPS-positive poorly differentiated cSCC (13/16, 81.3%) contrasting a low proportion of highly differentiated, non-metastasizing KA (1/9,11%). The findings of the current study correlate with those of Mehra et al. reporting 65% PD-L1 TPS-positive cSCC (30) and the study of Slater & Googe, demonstrating a correlation between PD-L1 TPS positivity at a ≥ 1% cut-off and the risk of metastatic disease (37). In line with Wu et al. (29), PD-L1 expression was low in most metastases and significantly lower compared with high-risk G3-SCC (p < 0.05) in the current study. CD8+ TIL, as an indicator for anti-tumour immune responses, have been associated with favourable prognosis in various solid cancers, including cSCC (39). In support of the immunogenicity of cSCC, CD8+ TIL were confirmed in all tissue specimens, with the majority of G3-cSCC (10/16) and metastases (4/5) displaying low CD8+ TIL density. Although the current study did not allow for an extended or functional characterization of immune infiltrates, evidence from preclinical models suggests that engagement of tumoural CD155 with TIGIT+ TIL impedes anti-tumour immune responses primarily by hampering CD8+ T-cell effector functions while augmenting Treg populations (17, 32, 35). In line with reported TIGIT expression on TIL, the current study analyses revealed a significantly higher CD8+ infiltration in TIGIT positive cSCC (p < 0.05) and a positive correlation of TIGIT immunoexpression with CD8+ TIL density in cSCC (p < 0.01). Tumoural CD155 expression has been linked to an unfavourable prognosis in various cancers (16, 19, 20, 31). While the design of the current study did not permit insight into the prognostic clinical value of iICP expression patterns, the current study analysis reveals significantly enhanced CD155 immunoexpression in high-risk G3-cSCC compared with lower grade cSCC (G1–G2, p < 0.05, data not shown) and to highly-differentiated, non-metastasizing KA (p < 0.01). To the best of our knowledge, TIGIT/CD155 immunoexpression has not yet been studied in KA. Due to its exceptionally benign, disparate biologic behaviour with spontaneous regression and extremely low potential for metastasis, a nosological position on the border between a benign tumour and a highly differentiated subtype of cSCC, is being debated for KA (21, 22). Confirming previous work (38), the current study observed dense CD8+ infiltrates in most KA (6/9). Yet, contrasting the strong positive correlation of TIGIT+ and CD8+ infiltrates in cSCC (p < 0.01), a trend towards a negative correlation between CD8+ and TIGIT+ infiltrates was notable in KA, potentially highlighting the relevance of iICP for modulating antitumoural responses in the TME. Furthermore, the current study reveals reverse iICP expression patterns in KA compared with high-risk G3-cSCC, with significantly higher TIGIT immunoexpression in KA compared with G3-cSCC (p < 0.001) and metastases (p < 0.05) opposing a significantly lower tumoural PD-L1 and CD155 expression in KA compared with G3-cSCC (PD-L1 TPS: p < 0.001, CD155: p < 0.01).
Combined biomarker approaches seem particularly relevant in view of the expanding repertoire of candidate iICP and therapeutics targeting TIGIT/CD155 in combination with anti-PD-1/PD-L1 are currently under pre-clinical and clinical investigation (35, 36).Notably, Lee et al. (40) recently reported that PD-L1 and CD155 co-expression determines sensitivity to PD-1 blockade in non-small-cell lung cancer. The current study found significantly higher CD155 immunoexpression in PD-L1 positive compared with PD-L1 negative tumours (p < 0.01) along with a strong positive CD155/PD-L1 TPS correlation in cSCC (p < 0.001) but not KA (p = 0.79). Furthermore, at a ≥ 5% cut-off, the vast majority of high-risk G3-cSCC displayed positivity for the CD155 ligand TIGIT (14/16, 87.5%) along with a non-significant trend towards higher TIGIT expression in PD-L1-negative cSCC (p = 0.08).
Study strengths and limitations
This study provides unique characteristics, such as stringent case selection and analysis of whole tumour sections, to account for intratumoural heterogeneity of iICP (24, 25). Nevertheless, this descriptive study has some limitations, including its retrospective design, limited sample size and lack of clinical outcome data. Since statistical analysis was exploratory, adjustments for multiple testing were not applied. Furthermore, cut-off-values for TIGIT/CD155 positivity are insufficiently established, though previous studies employed 5% and 1% cut-offs, respectively (20, 31, 32). Validation of the current findings in a larger series of samples, covering these specific aspects, is warranted.
Conclusion
This study provides a comprehensive insight into the collective landscape of the differential immunoexpression of PD-L1 and the novel co-inhibitory molecules TIGIT and CD155 along with CD8+ immune infiltrates across all differentiation stages of primary cSCC, metastases and in KA. Confirming upregulated tumoural PD-L1 expression in poorly differentiated, high-risk SCC, the current analysis further highlights TIGIT/CD155 signalling as potential complementary contributor to immune exhaustion in cSCC. While larger studies integrating clinical outcome data are warranted, these data support further exploration of the potential therapeutic value of combined targeting of these iICP and their exploitation as prognostic biomarkers for patients with advanced cSCC.
ACKNOWLEDGEMENTS
The authors thank the participating patients and staff of the Department of Dermatology at the University Hospital Heidelberg for their valuable contribution to this research, and Fabio Tabone, Biobank of the National Center for Tumor Diseases Heidelberg, for expert technical assistance.
The study was reviewed and approved by the Ethics Committee of the Medical Faculty of the University of Heidelberg (approval Nr. S-091/2011).
REFERENCES
- Karimkhani C, Boyers LN, Dellavalle RP, Weinstock MA. It’s time for “keratinocyte carcinoma” to replace the term “nonmelanoma skin cancer”. J Am Acad Dermatol 2015; 72: 186–187.
- Global Burden of Disease Cancer C, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 2019; 5: 1749–1768.
- Apalla Z, Nashan D, Weller RB, Castellsague X. Skin Cancer: Epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther (Heidelb) 2017; 7: 5–19.
- Schmults CD, Blitzblau R, Aasi SZ, Alam M, Andersen JS, Baumann BC, et al. NCCN Guidelines(R) Insights: squamous cell skin cancer, Version 1.2022. J Natl Compr Canc Netw 2021; 19: 1382–1394.
- Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. Eur J Cancer 2020; 128: 60–82.
- Cowey CL, Robert NJ, Espirito JL, Davies K, Frytak J, Lowy I, et al. Clinical outcomes among unresectable, locally advanced, and metastatic cutaneous squamous cell carcinoma patients treated with systemic therapy. Cancer Med 2020; 9: 7381–7387.
- Alberti A, Bossi P. Immunotherapy for cutaneous squamous cell carcinoma: results and perspectives. Front Oncol 2021; 11: 727027.
- Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 2017; 8: 561.
- Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 Blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018; 379: 341–351.
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015; 14: 561–584.
- Stratigos AJ, Garbe C, Dessinioti C, Lebbe C, Bataille V, Bastholt L, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur J Cancer 2020; 128: 83–102.
- Argenziano G, Fargnoli MC, Fantini F, Gattoni M, Gualdi G, Pastore F, et al. Identifying candidates for immunotherapy with cemiplimab to treat advanced cutaneous squamous cell carcinoma: an expert opinion. Ther Adv Med Oncol 2022; 14: 17588359211066272.
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17: e542–e551.
- Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016; 9: 5023–5039.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264.
- Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014; 26: 923–937.
- Manieri NA, Chiang EY, Grogan JL. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol 2017; 38: 20–28.
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009; 10: 48–57.
- Bronte V. The expanding constellation of immune checkpoints: a DNAMic control by CD155. J Clin Invest 2018; 128: 2199–2201.
- Albrecht T, Brinkmann F, Albrecht M, Lonsdorf AS, Mehrabi A, Hoffmann K, et al. programmed death ligand-1 (PD-L1) is an independent negative prognosticator in western-world gallbladder cancer. Cancers (Basel) 2021; 13: 1682.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol 2016; 74: 1220–1233.
- Selmer J, Skov T, Spelman L, Weedon D. Squamous cell carcinoma and keratoacanthomas are biologically distinct and can be diagnosed by light microscopy: a review. Histopathology 2016; 69: 535–541.
- Blessin NC, Simon R, Kluth M, Fischer K, Hube-Magg C, Li W, et al. Patterns of TIGIT expression in lymphatic tissue, inflammation, and cancer. Dis Markers 2019; 2019: 5160565.
- Nakamura S, Hayashi K, Imaoka Y, Kitamura Y, Akazawa Y, Tabata K, et al. Intratumoral heterogeneity of programmed cell death ligand-1 expression is common in lung cancer. PLoS One 2017; 12: e0186192.
- Rasmussen JH, Lelkaitis G, Hakansson K, Vogelius IR, Johannesen HH, Fischer BM, et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer 2019; 120: 1003–1006.
- Schildhaus HU. Der prädiktive Wert der PD-L1-Diagnostik. Pathologe 2018; 39: 498–519.
- Cohen EEW, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer 2019; 7: 184.
- Garcia-Diez I, Hernandez-Ruiz E, Andrades E, Gimeno J, Ferrandiz-Pulido C, Yebenes M, et al. PD-L1 expression is increased in metastasizing squamous cell carcinomas and their metastases. Am J Dermatopathol 2018; 40: 647–654.
- Wu S, Slater NA, Sayed CJ, Googe PB. PD-L1 and LAG-3 expression in advanced cutaneous squamous cell carcinomas. J Cutan Pathol 2020; 47: 882–887.
- Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 2018; 119: 153–159.
- Sun Y, Luo J, Chen Y, Cui J, Lei Y, Cui Y, et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma). Int Immunopharmacol 2020; 80: 106198.
- Wu L, Mao L, Liu JF, Chen L, Yu GT, Yang LL, et al. Blockade of TIGIT/CD155 signaling reverses t-cell exhaustion and enhances antitumor capability in head and neck squamous cell carcinoma. Cancer Immunol Res 2019; 7: 1700–1713.
- R Development Core T. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna 2014; https://www.R-project.org.
- Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2018; 17: 129.
- Thibaudin M, Limagne E, Hampe L, Ballot E, Truntzer C, Ghiringhelli F. Targeting PD-L1 and TIGIT could restore intratumoral CD8 T cell function in human colorectal cancer. Cancer Immunol Immunother 2022; 71: 2549–2563.
- Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology 2018; 7: e1466769.
- Slater NA, Googe PB. PD-L1 expression in cutaneous squamous cell carcinoma correlates with risk of metastasis. J Cutan Pathol 2016; 43: 663–670.
- Bauer C, Abdul Pari AA, Umansky V, Utikal J, Boukamp P, Augustin HG, et al. T-lymphocyte profiles differ between keratoacanthomas and invasive squamous cell carcinomas of the human skin. Cancer Immunol Immunother 2018; 67: 1147–1157.
- de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017; 6: e1356148.
- Lee BR, Chae S, Moon J, Kim MJ, Lee H, Ko HW, et al. Combination of PD-L1 and PVR determines sensitivity to PD-1 blockade. JCI Insight 2020; 5: e128633.