ORIGINAL REPORT
Effectiveness of Tofacitinib in Pre-adolescent Alopecia Areata: A Retrospective Case Series and Literature Review
Jundong HUANG, Tingting LI, Zixin TAN, Yan TANG, Ji LI, Fangfen LIU and Wei SHI
Department of Dermatology, Xiangya Hospital, Central South University, Hu Nan Key Laboratory of Aging Biology, Changsha, China
Alopecia areata (AA) is a common cause of hair loss in children. Despite numerous therapeutic options for paediatric AA, none have been found to be both effective and safe. Recent studies have demonstrated the efficacy and safety of the Janus kinase (JAK) inhibitor tofacitinib in adult patients with AA, whereas data on paediatric patients with AA in real-world practice are limited. This was a single-centre, retrospective study that included 11 pre-adolescent patients with AA treated with tofacitinib between December 2021 and September 2022. Clinical characteristics of patients, clinical response and adverse events were evaluated. Overall, 82% (9/11) of patients experienced hair regrowth and 64% (7/11) of patients experienced over 50% improvement in their Severity of Alopecia Tool (SALT) scores. Adverse events were mild. In the literature, tofacitinib has been used to treat AA in 31 children ≤12 years of age who failed to respond to prior treatments. Eighty-seven percent (27/31) of these patients showed significant responses based on changes in their SALT scores. This case series demonstrates that oral tofacitinib is an effective and safe treatment option for paediatric AA, particularly for children who have failed to respond to traditional treatments or are not suitable for such treatments.
Key words: alopecia areata; tofacitinib; pre-adolescent; paediatric dermatology; treatment.
SIGNIFICANCE
Recent studies have demonstrated the efficacy and safety of tofacitinib, a Janus kinase inhibitor, in adult patients with alopecia areata, whereas data on paediatric patients with alopecia areata in real-world practice are limited. This retrospective case series evaluated the efficacy of tofacitinib in 11 children with alopecia areata. Overall, clinical improvement was demonstrated in 9 patients (82%). In addition, a literature review was performed and 31 cases of pre-adolescent alopecia areata treated with tofacitinib were analysed. The results demonstrate that oral tofacitinib in the treatment of paediatric alopecia areata has significant clinical efficacy and safety, providing a novel alternative for children with alopecia areata who fail to respond to, or who cannot tolerate, traditional treatment.
Citation: Acta Derm Venereol 2023; 103: adv13418. DOI https://doi.org/10.2340/actadv.v103.13418.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Aug 24, 2023; Published: Sep 20, 2023
Corr: Fangfen Liu and Wei Shi, Department of Dermatology, Xiangya Hospital, Central South University,Hu Nan Key Laboratory of Aging Biology, Changsha, China. E-mails: 776407936@qq.com; shiwei@csu.edu.cn
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Alopecia areata (AA) is a non-scarring, immune-mediated alopecia that can occur at any age (1). It is the most common cause of hair loss in paediatric patients; approximately half of reported cases occur before the age of 20 years (2). The natural history of AA is variable and a considerable proportion of patients with chronic AA (defined as the current episode persisting beyond 1 year) will eventually develop alopecia totalis or alopecia universalis (3, 4). The psychological impact of AA on children can be significant due to its persistent and recurrent nature, and can lead to a reduced quality of life (5). Early intervention is crucial, as prolonged disease duration and early onset can lead to poor treatment response (6). However, managing and treating paediatric AA can be challenging due to the lack of randomized controlled trials and limited effective systemic therapies with high adverse effects (7, 8).
Topical therapy (with corticosteroids, minoxidil) and contact immunotherapy are the usual management options for paediatric AA, with systemic therapy reserved for severe or refractory cases. Recent studies have shown that the Janus kinase (JAK)/Signal Transducer and Activator of Transcription (STAT) pathway plays a crucial role in the immunopathogenesis of AA (9); and tofacitinib, a JAK 1/3 inhibitor, has emerged as a potential alternative treatment for refractory cases of AA. Several studies have demonstrated the encouraging efficacy and well-tolerated safety of tofacitinib in adult patients with AA, especially in those who have previously failed other systemic treatments (10, 11). Although not approved for use in paediatric AA, a few case reports and studies have shown promising results in children treated with tofacitinib (6, 12–18).
However, evidence on the use of oral tofacitinib for pre-adolescent AA is still limited. This retrospective case series evaluated the efficacy of tofacitinib in 11 children with AA. This study also reviewed the literature on pre-adolescent AA treated with tofacitinib.
METHODS
Patients
Data were collected on patients aged from 6 to 12 years with AA who were treated with tofacitinib between December 2021 and September 2022 in Xiangya Hospital Central South University, Changsha, China. The diagnosis of AA was confirmed by at least 2 dermatologists based on typical skin manifestations and dermoscopy. All patients had parental consent for tofacitinib use and were made aware of its off-label status for use in AA.
Demographic and clinical information, including age, sex, weight, age of onset, disease duration, severity of alopecia, extra-scalp manifestation, duration of the current episode of hair loss (defined as beginning with when last had normal complement of scalp hair excluding hair loss from etiologies other than AA), family history, prior treatments, and comorbidity were recorded.
Treatment
Prior to the treatment, each patient was assessed to exclude patients with contraindications, including routine laboratory investigations (complete blood count, serum biochemical), infection-related indicators (T-cell spot test for tuberculosis, HIV antibody test, HBsAg, HCV antibody, T. pallidum particle agglutination test, chest computed tomography (CT) scan).
All 11 patients (none of whom had any other health issues) were administered tofacitinib according to their weight (19, 20), with an initial dose of 5 mg twice a day (weight > 40 kg) or 5 mg per day (weight < 40 kg). Regular follow-up laboratory testing (including complete and differential count, lipid profiles, and liver and renal functions) was conducted in patients who continued tofacitinib treatment.
Efficacy evaluation
The Pediatric-specific Severity of Alopecia Tool (pSALT) (21) was assessed at each clinic visit. Treatment response was assessed by calculating the percentage improvement in pSALT score from baseline to most recent evaluation. Treatment response was defined as: response (the pSALT score decreased > 5%); significant response (> 50%); or complete response (100%).
Literature review
A comprehensive literature search of PubMed was performed in March 2023. Search terms included “alopecia areata”, “alopecia circumscripta”, “alopecia totalis”, “alopecia universalis”, “alopecia celsi”, “ophiasis”, “nonscarring hair loss”, “tofacitinib”, “Xeljanz”, “tasocitinib”, “tofacitinib citrate”, “pediatric”, “children”, and “child age group”. Inclusion criteria were: English language studies in which: (i) patients received oral tofacitinib as treatment; and (ii) hair regrowth rate measured with Severity of Alopecia Tool (SALT) score. Exclusion criteria were: (i) studies with patients aged > 12 years; (ii) reviews and editorials; and (iii) studies in which no patient details were given.
RESULTS
Patient characteristics
Eleven patients with AA were treated with tofacitinib for a median duration of 6 months (range 3-10 months). A detailed description of these 11 cases is shown in Table I. Six children were boys and 5 children were girls, age range 7–12 years, mean ± standard deviation (SD) age 9.73 ± 2.28 years. Mean ±SD duration of AA was 44.09 ± 38.14 months. The mean ±SD duration of the current episode of alopecia was 38.55 ± 39.8 months. All patients reached the diagnostic criteria for severe AA at baseline and had failed to respond to topical corticosteroids. Some patients had also failed to respond to other treatments (intralesional corticosteroids (1 patient); topical minoxidil (6 patients); prednisone (6 patients); systemic immunomodulatory agents including cyclosporine (3 patients); and methotrexate (1 patient)). Two patients had comorbid atopic dermatitis.
Evaluation of treatment outcome
No active or latent infections or other significant metabolic disturbances were found. All patients were treated with tofacitinib monotherapy. The initial treatment dose was 10 mg (weight > 40 kg) or 5 mg (weight < 40 kg) daily. The dose was increased every 1–3 months in patients based on treatment response and tolerability.
All 11 patients completed a minimum of 3 months of treatment. Eight patients (73%) experienced hair regrowth (responders): 1 responder (patient 5) had complete regrowth over 7 months while taking tofacitinib 5 mg daily, but then relapsed after 2 months of discontinuation. Three out of 11 patients were non-responders: 2 patients (18%) did not respond to tofacitinib despite attempts to increase the dose and extend the duration of the treatment. One patient experienced very minimal regrowth (and was therefore characterized as a non-responder). Among all patients, the median percentage change in pSALT score was 77% (mean 61%; range 0–100%). Among responders, the median percentage change in SALT score was 90.5% (mean 83.5%; range 45%–100%). Representative photographs of patients (patient 2, patient 3, and patient 9) who responded to therapy are shown in Figs 1–3.
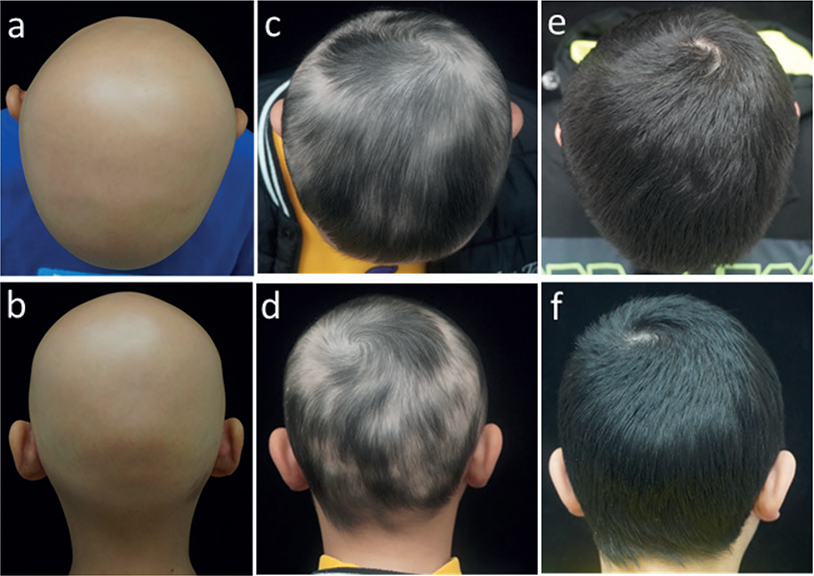
Fig. 1. Tofacitinib treatment response in an 11-year old boy with alopecia areata (AA). (a, b) Baseline; (c, d) 2 months; (e, f) 4 months.
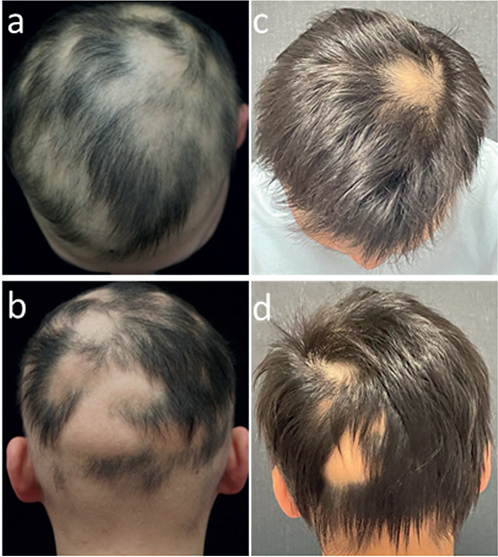
Fig. 2. Tofacitinib treatment response in patient 3. (a, b) Baseline; (c, d) 4 months.
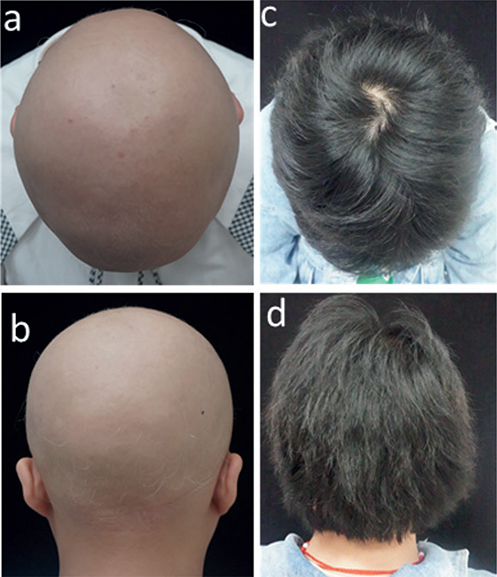
Fig. 3. Tofacitinib treatment response in patient 9. (a, b) Baseline; (c, d) 6 months.
Adverse events were mild and limited to mild increases in liver transaminase levels (patient 6), low haemoglobin (patient 7), folliculitis and elevated uric acid (patient 1). No side-effects such as opportunistic infection, neutropaenia, thrombotic events, or tuberculosis were reported during follow-up.
DISCUSSION
AA is one of the most common causes of hair loss disorders in children. Although there are many therapeutic options for paediatric AA, none are satisfactory in terms of effectiveness and safety. Parents are usually reluctant to use drugs that are harmful to their children’s mental and physical development, such as oral corticosteroids or immunosuppressants, resulting in a treatment stalemate. This retrospective study presents an evaluation of the usage of tofacitinib for paediatric AA at a large tertiary hospital, emphasizing the efficacy and safety of tofacitinib in practice. Overall, 73% of patients experienced hair regrowth and 64% of patients experienced over 50% improvement in their SALT scores, which is similar to the change reported by Rebekka et al. (6), but higher than the change reported in adult patients (20, 22). During follow-up, the reported adverse events were mild and tolerable.
To date, the mechanisms leading to AA are not fully understood, but probably involve a combination of genetic predisposition and environmental triggers, which may disturb the immune balance of the follicle niche and destroy the immune privilege, eventually leading to auto-immune attack on the follicle bulb cells, mediated by T cells (9). The positive feedback loop of interferon (IFN)-gamma and interleukin (IL)-15 activate target immune cells via the JAK-STAT signalling pathway. The importance of this pathway is illustrated by the efficacy of JAK inhibitors for AA in recent studies (10, 11).
Although tofacitinib was the first JAK inhibitor to be used in the treatment of AA, and its efficacy was encouraging, no further randomized controlled trials were conducted to verify its actual efficacy. Based on the long-term monitoring results of tofacitinib usage in patients with rheumatoid arthritis, concerns regarding the long-term therapy side-effects limited its clinical application, especially in the paediatric population (19). However, a recent clinical trial assessing the efficacy of tofacitinib in paediatric patients (2–17 years) with juvenile idiopathic arthritis provided some referenced findings about short-term safety of tofacitinib for children (23). There were no new potential safety risks identified and the adverse events occurred in the entire study period seemed to be mild, self-limited, and acceptable. It is notable that the incidence of upper respiratory infections (URI) in the current study is lower than reported previously (20); a possible reason may be the increasing use of face masks and fewer trips outdoors, due to measures for preventing the transmission of SARS-CoV-2 (COVID-19) in China since 2020. In addition, an ongoing, long-term extension trial (NCT01500551, https://classic.clinicaltrials.gov/ct2/show/NCT01500551?term=01500551&draw=2&rank=1) might be significant in observing the long-term safety of tofacitinib in paediatric patients.
The comprehensive literature search on PubMed initially yielded 16 articles. After applying inclusion and exclusion criteria, 8 articles were included for analysis. The articles include case series and case reports, but lack prospective studies. Patient characteristics and treatment and response data are shown in Table II. The total number of participants was 31, with an age range of 3–12 years and a disease duration range of 1–6 years.
| Study number. | Author | Year | Total patient* | Sex (M/F) | Age, years Median (range) | Duration of disease, years (range) | Prior treatment | Initial pSALT score (%) Median (range) | Treatment duration, months (range) [daily dosage] | pSALT improvement Median (range) | Side-effects |
| 1 | Leslie CS13 | 2017 | 8 (1) | NA | 12 | 4 | TI, TCS, OCS | 100 | 6 [10 mg] | 62 | N |
| 2 | Liza B, et al12 | 2018 | 1 (1) | 1 (M)/0 (F) | 8 | NA | TCS, ILC | 100 | 6 [10 mg] | 100 | Headache |
| 3 | Brittany G, et al14 | 2018 | 4 (4) | 1 (M)/3 (F) | 9 (8–10) | 3 (2–5) | TI, TCS, OCS, CsA, PRP, topical minoxidil, topical tofacitinib, NBUVB, ustekinumab | 100 (100) | 6–15 [5–10 mg] | 81 (1–100) | N |
| 4 | Dai YX, et al15 | 2019 | 3 (3) | 2 (M)/1 (F) | 4 (4–5) | 1 (1–3) | TI, TCS, ILC | 100 (100) | 6–20 [2.5–5 mg] | 59 (50–94) | Diarrhoea (n = 1), URI (n = 2) |
| 5 | Rebekka J, et al6 | 2020 | 14 (11) | 5 (M)/6 (F) | NA | 2.5 (1–6) | TI, TCS, ILC, OCS, CsA, AZA, oral minoxidil, topical tofacitinib, topical bimatoprost | 74 (6–100) | 7–38 [2.5–15 mg] | 67.7 (–400–100) | URI (n = 3), elevation in AST/ALT (n = 5), eosinophilia (n = 5), hypercholesterolaemia (n = 3), elevated urea (n = 3), hyperkalaemia (n = 3), low total protein (n = 4), elevated triglycerides (n = 1), hyperbilirubinaemia (n = 1) |
| 6 | Kibbie J, et al17 | 2021 | 11 (1) | 0 (M)/1 (F) | 8 | 6 | TCS | 100 | 20 [10 mg] | > 50 | N |
| 7 | Geng SL, et al16 | 2022 | 5 (5) | 2 (M)/3 (F) | 3 (3–5) | 2 (0.6–3) | TI, TCS, OCS, | 100 (75–100) | 4–8 [5 mg] | 100(85–100) | Elevation in ALT/AST (n = 2) |
| 8 | Youssef S, et al18 | 2022 | 10 (5) | 2 (M)/3 (F) | 12 (7–12) | NA | TCS, ILC, OCS | 90 (40–100) | 1–9 [5–15 mg] | 100 (60–100) | N |
| *Patients meeting the age requirement are shown in brackets | |||||||||||
| pSALT: Pediatric-specific Severity of Alopecia Tool; TI: topical immunotherapy; TCS: topical corticosteroids; OCS: oral corticosteroids; ILC: intralesional corticosteroids; URI: upper respiratory infection; PRP: platelet-rich plasma; NBUVB: narrowband ultraviolet B phototherapy; CsA: cyclosporine; AZA: azathioprine; N: none; NA: not available; M: male; F: female. | |||||||||||
The literature review found several studies that proved the efficacy of tofacitinib in paediatric patients with AA who failed to respond to conventional topical therapies or other systemic agents. In general, 87% of the studied patients (27/31) showed significant responses based on SALT score changes, consistent with the findings in the current study cohort (82%). The duration of treatment ranged from 6 to 38 months. Two patients failed to respond to tofacitinib therapy with a mean treatment duration of 8 months, while the other 2 patients experienced mild hair regrowth. A total of 34 adverse events (AEs) were reported. The most frequently observed AEs were URI (14.7%), elevated liver enzymes alanine aminotransferase/aspartate aminotransferase (14.7%), and eosinophilia (14.7%). All AEs were mild and predictable and none of the patients stopped taking the drug because of AEs.
The current literature suggests that hair regrowth associated with tofacitinib treatment did not appear to be long-lasting (20). One hypothesis is that tofacitinib may inhibit IL-10 signalling while treating AA, negating part of the actual therapeutic effects or increasing sensitivity to the loss of immune status of follicles (24, 25). Relapse occurred after cessation of tofacitinib in 1 of our patients, which indicated that maintenance therapy or an extension of treatment duration might be necessary for obtaining continued remission.
Limitations include a small sample size, retrospective nature, lack of a control group, and short follow-up period. However, the current results supplement the knowledge gap in the treatment of paediatric AA and provide data to guide choice of systemic therapy.
In conclusion, this retrospective case series demonstrates that oral tofacitinib in the treatment of paediatric AA has significant clinical efficacy and safety, providing a novel alternative for children with AA who fail to respond to, or who cannot tolerate, traditional treatment. Further studies and clinical trials are needed to evaluate the long-term safety of tofacitinib in children, and the biomarkers of therapeutic efficacy.
ACKNOWLEDGEMENTS
The study was approved by the institutional research ethics boards of Xiangya Hospital, Central South University, Changsha, China; approval number: 202303045.
The study data will be shared on reasonable request to the corresponding author.
REFERENCES
- Gilhar A, Etzioni A, Paus R. Alopecia areata. New Engl J Med 2012; 366: 1515–1525.
- Kyriakis KP, Paltatzidou K, Kosma E, Sofouri E, Tadros A, Rachioti E. Alopecia areata prevalence by gender and age. J Eur Acad Dermatol 2009; 23: 572–573.
- Cranwell WC, Lai VW, Photiou L, Meah N, Wall D, Rathnayake D, et al. Treatment of alopecia areata: an Australian expert consensus statement. Austral J Dermatol 2019; 60: 163–170.
- Lai V, Sinclair R. Utility of azathioprine, methotrexate and cyclosporine as steroid-sparing agents in chronic alopecia areata: a retrospective study of continuation rates in 138 patients. J Eur Acad Dermatol 2020; 34: 2606–2612.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol 2016; 75: 806–812.
- Jerjen R, Meah N, Trindade DCL, Wall D, Eisman S, Sinclair R. Treatment of alopecia areata in pre-adolescent children with oral tofacitinib: a retrospective study. Pediatr Dermatol 2021; 38: 103–108.
- Barton VR, Toussi A, Awasthi S, Kiuru M. Treatment of pediatric alopecia areata: a systematic review. J Am Acad Dermatol 2022; 86: 1318–1334.
- Afford R, Leung A, Lam JM. Pediatric alopecia areata. Curr Pediatr Rev 2021; 17: 45–54.
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol 2022; 13: 955035.
- Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol 2017; 76: 22–28.
- Park HS, Kim MW, Lee JS, Yoon HS, Huh CH, Kwon O, et al. Oral tofacitinib monotherapy in Korean patients with refractory moderate-to-severe alopecia areata: a case series. J Am Acad Dermatol 2017; 77: 978–980.
- Brown L, Skopit S. An excellent response to tofacitinib in a pediatric alopecia patient: a case report and review. J Drugs Dermatol 2018; 17: 914–917.
- Castelo-Soccio L. Experience with oral tofacitinib in 8 adolescent patients with alopecia universalis. J Am Acad Dermatol 2017; 76: 754–755.
- Craiglow BG, King BA. Tofacitinib for the treatment of alopecia areata in preadolescent children. J Am Acad Dermatol 2019; 80: 568–570.
- Dai YX, Chen CC. Tofacitinib therapy for children with severe alopecia areata. J Am Acad Dermatol 2019; 80: 1164–1166.
- Geng SL, Gong T, Ji C, Su HH. Oral tofacitinib for successful treatment of refractory alopecia areata in preschool children. J Eur Acad Dermatol 2022; 36: e1055–e1057.
- Kibbie J, Kines K, Norris D, Dunnick CA. Oral tofacitinib for the treatment of alopecia areata in pediatric patients. Pediatr Dermatol 2022; 39: 31–34.
- Youssef S, Bordone LA. Clinical response to oral tofacitinib in pediatric patients with alopecia areata. JAAD Case Rep 2023; 31: 83–88.
- Nash P, Kerschbaumer A, Dorner T, Dougados M, Fleischmann RM, Geissler K, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis 2021; 80: 71–87.
- Guo L, Feng S, Sun B, Jiang X, Liu Y. Benefit and risk profile of tofacitinib for the treatment of alopecia areata: a systemic review and meta-analysis. J Eur Acad Dermatol 2020; 34: 192–201.
- Bernardis E, Nukpezah J, Li P, Christensen T, Castelo-Soccio L. Pediatric severity of alopecia tool. Pediatr Dermatol 2018; 35: e68–e69.
- Hogan S, Wang S, Ibrahim O, Piliang M, Bergfeld W. Long-term treatment with tofacitinib in severe alopecia areata: an update. J Clin Aesthet Dermatol 2019; 12: 12–14.
- Ruperto N, Brunner HI, Synoverska O, Ting TV, Mendoza CA, Spindler A, et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet 2021; 398: 1984–1996.
- Freyschmidt-Paul P, McElwee KJ, Happle R, Kissling S, Wenzel E, Sundberg JP, et al. Interleukin-10-deficient mice are less susceptible to the induction of alopecia areata. J Invest Dermatol 2002; 119: 980–982.
- McElwee KJ, Hoffmann R, Freyschmidt-Paul P, Wenzel E, Kissling S, Sundberg JP, et al. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4+ and CD8+ cells. J Invest Dermatol 2002; 119: 1426–1433.