ORIGINAL REPORT
Clinically Meaningful Responses to Dupilumab Among Children Aged 6 Months to 5 Years with Moderate-to-severe Atopic Dermatitis who did Not Achieve Clear or Almost Clear Skin According to the Investigator’s Global Assessment: A Post Hoc Analysis of a Phase 3 Trial
Michael J. CORK1,2, Benjamin LOCKSHIN3,4, Andreas PINTER5, Zhen CHEN6, Brad SHUMEL6 and Randy PRESCILLA7
1Sheffield Children’s Hospital, 2Sheffield Dermatology Research, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, UK, 3US Dermatology Partners, Rockville, MD, 4Georgetown University, Washington, DC, USA, 5Department of Dermatology, University Hospital Frankfurt am Main, Frankfurt am Main, Germany, 6Regeneron Pharmaceuticals Inc., Tarrytown, NY and 7Sanofi, Cambridge, MA, USA
In young children, atopic dermatitis (AD) imposes a multidimensional burden on many aspects of their quality of life (QoL) and that of their families. LIBERTY AD PRESCHOOL part B was a randomized, double-blinded, placebo-controlled phase 3 trial in 162 children (aged 6 months to 5 years) with moderate-to-severe AD receiving dupilumab or placebo, plus low-potency topical corticosteroids. Post hoc analyses were performed on the full analysis set (FAS) and a subset of patients with Investigator’s Global Assessment score > 1 at week 16. The primary outcome was the proportion of patients at week 16 achieving a composite endpoint encompassing clinically meaningful changes in AD signs, symptoms and QoL: ≥ 50% improvement in Eczema Area and Severity Index; and/or ≥ 4-point reduction in worst scratch/itch numerical rating scale; and/or ≥ 6-point reduction in Children’s Dermatology Life Quality Index/Infants’ Dermatitis Quality of Life Index. Significantly more patients receiving dupilumab vs placebo achieved the composite endpoint in both the FAS (77.7% vs 24.6%, p < 0.0001) and subgroup (68.9% vs 21.5%, p < 0.0001). Dupilumab provided rapid and significant, clinically meaningful improvements in AD signs, symptoms, and QoL in the overall group and subgroup of patients who did not achieve clear or almost clear skin at week 16.
SIGNIFICANCE
The LIBERTY AD PRESCHOOL study evaluated the safety and efficacy of dupilumab in children aged 6 months to 5 years with moderate-to-severe atopic dermatitis. This analysis focused on the subgroup of patients who did not achieve clear or almost clear skin after 16 weeks of treatment. Because atopic dermatitis is a complex condition that causes severe skin disease, itch, and reduced quality of life, a complex measure was used to analyse these 3 aspects. Using this measure, after 16 weeks of dupilumab treatment, a majority of patients in the subgroup had improvements in atopic dermatitis that are considered meaningful to patients.
Key words: atopic dermatitis; eczema; dupilumab; infants; paediatrics; quality of life.
Citation: Acta Derm Venereol 2024; 104: adv13467. DOI https://doi.org/10.2340/actadv.v104.13467.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Jun 8, 2023; Accepted: Oct 18, 2023; Published: Feb 12, 2024
Corr: Michael J. Cork, Sheffield Dermatology Research, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, UK. E-mail: M.J.Cork@sheffield.ac.uk
Competing interests and funding: MJC reported grants from Hyphens Pharma, Johnson & Johnson, Kymab, LEO Pharma, L’Oréal, Perrigo, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi during the conduct of the study; personal fees from AbbVie, Astellas Pharma, Boots, Dermavant, Eli Lilly, Galapagos, Galderma, Hyphens Pharma, Johnson & Johnson, LEO Pharma, L’Oréal, Menlo Therapeutics, Novartis, Oxagen, Perrigo, Pfizer, Procter & Gamble, Reckitt Benckiser, Regeneron Pharmaceuticals Inc., and Sanofi during the conduct of the study. BL reported personal fees from AbbVie, Eli Lilly, Incyte, LEO Pharma, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi outside the submitted work; is an investigator and speaker for Eli Lilly and Regeneron Pharmaceuticals Inc.; is an investigator for Anacor Pharmaceuticals, Dermira, Franklin Bioscience, and LEO Pharma; and is an investigator, speaker, and consultant for AbbVie. AP reported payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Almirall Hermal, Amgen, Biogen Idec, BioNTech, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Galderma, GSK, Hexal, Janssen, LEO Pharma, MC2 Pharma, Medac, Merck-Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Regeneron Pharmaceuticals Inc., Roche, Sandoz, Sanofi, Schering-Plough, Tigercat Pharma, and UCB; participation in a data and safety monitoring board or advisory board for AbbVie, Almirall Hermal, Amgen, Biogen Idec, BioNTech, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Galderma, GSK, Hexal, Janssen, LEO Pharma, MC2 Pharma, Medac, Merck-Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Regeneron Pharmaceuticals Inc., Roche, Sandoz, Sanofi, Schering-Plough, Tigercat Pharma, and UCB. ZC and BS are employees and shareholders of Regeneron Pharmaceuticals Inc. RS is an employee of Sanofi and may hold and/or stock options in the company.
INTRODUCTION
Atopic dermatitis (AD) is a chronic type 2 inflammatory skin disease that typically presents with eczematous lesions and is associated with intense pruritus (1). Infants and young children with moderate-to-severe AD have a multidimensional disease burden, with skin lesions, intense itching, and disturbed sleep, which can have a profoundly negative impact on their quality of life (QoL) and that of their caregivers and families (2–4).
For children who have an inadequate response to topical therapies for AD, there are limited systemic treatment options available. In children younger than 6 years of age, although systemic glucocorticoids are sometimes used, they are not recommended by treatment guidelines (5).
Dupilumab, a fully human VelocImmune®-derived (6, 7) monoclonal antibody, blocks the shared receptor subunit for interleukin (IL)-4 and IL-13, thus inhibiting signalling of both IL-4 and IL-13. Multiple clinical trials of dupilumab in children, adolescents, and adults have demonstrated rapid, significant, and sustained improvements in AD signs and symptoms, and QoL, with an acceptable safety profile (8–13).
The LIBERTY AD PRESCHOOL part B study, a phase 3 placebo-controlled trial in children aged 6 months to 5 years with moderate-to-severe AD, showed significant improvements in AD signs, symptoms, and QoL in children receiving dupilumab with concomitant low-potency topical corticosteroids (TCS). As required by regulatory authorities, the primary endpoint in that trial was achievement of an Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear skin) (12, 14, 15). Although IGA is widely used because of its simplicity and convenience in the assessment of skin signs, it does not take into account the multidimensional aspects of AD impacting the patient’s QoL, including symptoms such as itch, and the body surface area (BSA) affected by AD lesions. Composite measures that include evaluation of diverse aspects of AD and the patient’s own assessment of their condition, including signs, symptoms, and QoL, may provide a more comprehensive picture of treatment effects. Previous analyses of clinical trial data have shown that dupilumab treatment provides rapid, significant, and sustained improvements in AD signs, symptoms, and QoL in adults (16), adolescents (17), and children aged 6–11 years (18), not only in the overall study population (full analysis set; FAS) but also in the subgroup of patients who did not achieve an IGA score of 0 or 1 at week 16.
This post hoc analysis of data from the LIBERTY AD PRESCHOOL part B trial aimed to assess the impact of dupilumab vs placebo on clinically meaningful improvements in AD signs, symptoms, and QoL in children aged 6 months to 5 years with moderate-to-severe AD in the FAS, and in the subgroup of patients who did not achieve an IGA score of 0 or 1 at week 16.
MATERIALS AND METHODS
Study design and patients
The LIBERTY AD PRESCHOOL part B study was a randomized, placebo-controlled, double-blinded, parallel-group, phase 3 study conducted in accordance with the ethical standards of the responsible committees and the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice. Full details of the study design have been reported previously (12). Briefly, patients aged 6 months to 5 years with moderate-to-severe AD (defined as an IGA score ≥ 3) inadequately controlled by topical medications, or for whom topical therapy was not advisable, were enrolled. Patients were randomized (1:1) to receive either subcutaneous dupilumab every 4 weeks (q4w: 200 mg for baseline bodyweight 5 to < 15 kg; 300 mg for baseline bodyweight 15 to < 30 kg) or matched placebo, plus a standardized regimen of low-potency TCS for 16 weeks. This post hoc analysis included data for the FAS (all randomized patients) and the subgroup of patients with IGA score > 1 at week 16 (IGA > 1 subgroup).
Study outcomes
The primary outcome of the LIBERTY AD PRESCHOOL part B trial was the proportion of patients with an IGA score of 0 or 1 at week 16. In this post hoc analysis of the trial data, the primary outcome was the proportion of patients achieving a composite endpoint of the following: ≥ 4 points reduction in Worst Scratch/Itch Numerical Rating Scale (WSI-NRS); and/or ≥ 6 points reduction in Children’s Dermatology Life Quality Index (CDLQI; for patients aged ≥ 4 years) or Infants’ Dermatitis Quality of Life Index (IDQoL; for patients aged < 4 years); and/or a ≥ 50% improvement in Eczema Area and Severity Index (EASI-50), all from baseline. Other clinician- and patient-reported outcomes included change from baseline to week 16 in the following: EASI; Scoring Atopic Dermatitis (SCORAD) total score; CDLQI or IDQoL; BSA affected by AD; WSI-NRS score; Skin Pain Numerical Rating Scale (NRS) score; Global Individual Signs Score (GISS) total; Dermatitis Family Impact (DFI), a measure of the impact of a child with AD on the QoL of adult members of their family; and Patient-Oriented Eczema Measure (POEM). This study also assessed the proportion of patients achieving EASI-50, ≥ 75%/90% improvement from baseline in EASI (EASI-75/EASI-90); ≥ 50% improvement in SCORAD (SCORAD-50); ≥ 3-or 4-point improvement in WSI-NRS score; ≥ 6-point improvement in POEM score; ≥ 6-point improvement in CDLQI/IDQoL; and “no” or “mild symptoms” assessed by the Caregiver Global Impression of Disease Severity (CGID), a 5-point scale (“no”, “mild”, “moderate”, “severe”, or “very severe”) on which caregivers evaluate the severity of a child’s AD symptoms during the previous 7 days. Clinically meaningful improvements in the assessed scales were defined as ≥ 3-point reduction in WSI-NRS from baseline, ≥ 6-point reduction in POEM from baseline, and ≥ 6-point reduction in CDLQI/IDQoL from baseline, based on published data for children (19–21). The use of rescue medication was also evaluated.
Safety outcomes were assessed in the safety analysis set (SAS, defined as all patients who received any study drug) and in the IGA > 1 subgroup. Safety outcomes included proportions of patients with ≥ 1 treatment-emergent adverse event (TEAE), ≥ 1 serious TEAE, and TEAEs leading to permanent withdrawal from the study.
Statistical analysis
Descriptive statistics were used to assess demographic and clinical characteristics. Least squares mean percentage change from baseline was reported for EASI, SCORAD, WSI-NRS, POEM, CDLQI/IDQoL, DFI, Skin Pain NRS, and GISS total. The proportions of patients meeting pre-specified thresholds for other outcomes were reported as number and percentage of total.
A Cochran–Mantel–Haenszel test was used to analyse categorical endpoints, with adjustment for randomization strata. Values after first rescue treatment used were set to missing. Patients with missing values at week 16 due to rescue treatment, withdrawn consent, adverse events (AEs), or lack of efficacy (as deemed by the investigator) were considered non-responders. Patients with missing values due to other reasons, including COVID-19 (SARS-CoV-2), were imputed by multiple imputation (MI), and the response status was then derived.
Continuous endpoints were analysed using analysis of covariance, with treatment group, stratification factors, and relevant baseline measurements included in the model. Patients with missing values at week 16 due to rescue treatment, withdrawn consent, AEs, or lack of efficacy were imputed by worst observation carried forward, or the baseline value was used if there were no post-baseline values. Patients with missing values due to other reasons, including COVID-19, were imputed by MI.
RESULTS
Patients
A total of 162 patients in the FAS were randomized to receive dupilumab 200 or 300 mg q4w plus low potency TCS (n = 83), or placebo q4w plus low potency TCS (n = 79); 136 patients had an IGA score > 1 at week 16 (60 dupilumab; 76 placebo) and were included in the IGA > 1 subgroup.
Baseline demographics and clinical characteristics of patients in the FAS and in the IGA > 1 subgroup were generally similar across treatment groups (Table SI). A majority of patients (≥ 80%) across treatment groups had at least 1 comorbid allergic condition at baseline.
Clinician- and patient-reported outcomes
In the IGA > 1 subgroup, significantly more patients receiving dupilumab compared with placebo achieved the composite endpoint (EASI-50, and/or change in WSI-NRS > 4, and/or change in CDLQI/IDQoL > 6) at week 16: 68.9% vs 21.5%, respectively; p < 0.0001. Significant improvements were evident as early as week 2 and sustained through week 16 (Fig. 1). Similar results were observed in the FAS. Dupilumab-treated patients in both the IGA > 1 subgroup and FAS showed greater improvements from baseline to week 16 in absolute mean values of the individual measures included in the composite endpoint (EASI, WSI-NRS, and CDLQI/IDQoL) (Fig. 2).
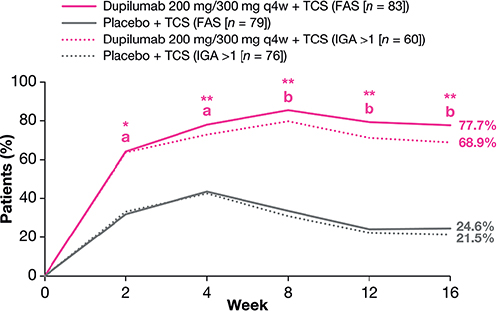
Fig. 1. Proportions of patients achieving the composite endpoint of 50% improvement in Eczema Area and Severity Index and/or change in Worst Scratch/Itch Numerical Rating Scale ≥ 4, and/or change in Children’s Dermatology Life Quality Index/Infants’ Dermatitis Quality of Life Index ≥ 6 over time (full analysis set (FAS) and Investigator’s Global Assessment (IGA) > 1 subgroup). ap < 0.01 vs placebo, bp < 0.0001 vs placebo (for IGA > 1 subgroup). q4w: every 4 weeks; TCS: topical corticosteroids; *p < 0.01 vs placebo, **p < 0.0001 vs placebo (for FAS).
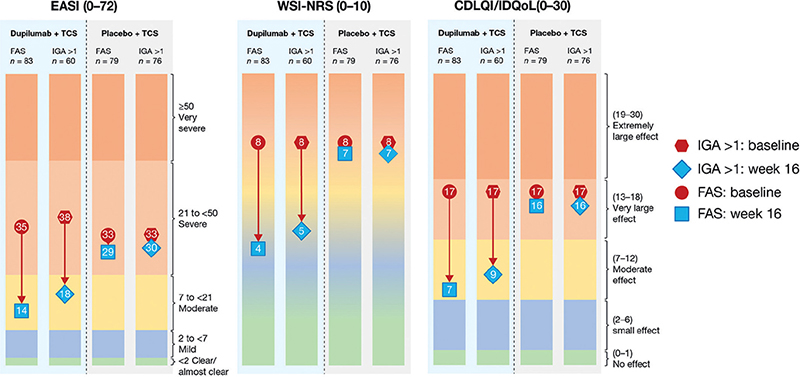
Fig. 2. Change in mean Eczema Area and Severity Index (EASI) score, mean Worst Scratch/Itch Numerical Rating Scale (WSI-NRS) score, and mean Children’s Dermatology Life Quality Index/Infants’ Dermatitis Quality of Life Index (CDLQI/IDQoL) score from baseline to week 16 (full analysis set (FAS) and Investigator’s Global Assessment (IGA) > 1 subgroup). The colour scale graphic displays the changes in absolute values from baseline (red) to week 16 (blue) for each outcome. EASI ranges from < 2 = clear/almost clear; 2 to < 7 = mild; 7 to < 21 = moderate; 21 to < 50 = severe; ≥ 50 = very severe; CDLQI and IDQoL range from 0 to 1 = no effect; 2 to 6 = small effect; 7 to 12 = moderate effect; 13 to 18 = very large effect; 19 to 30 = extremely large effect; WSI-NRS ranges from 0 = no itch (green zone) to 10 = worst itch imaginable (orange zone). TCS: topical corticosteroids.
More patients receiving dupilumab vs placebo achieved clinically meaningful improvements in all 3 of the individual measures included in the composite endpoint in the IGA > 1 subgroup (31.2% vs 4.3%, respectively; Fig. 3). Similar results were observed in the FAS. In addition, in both the IGA > 1 subgroup and the FAS, more patients receiving dupilumab vs placebo achieved clinically meaningful improvements in 2 out of the 3 measures.
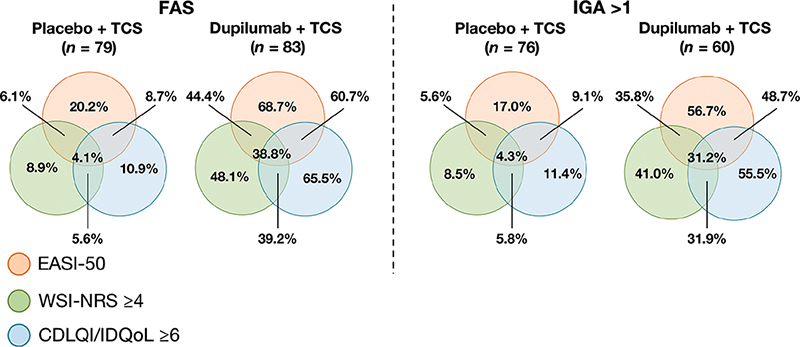
Fig. 3. Proportions of patients who achieved 50% improvement in Eczema Area and Severity Index (EASI), change in Worst Scratch/Itch Numerical Rating Scale (WSI-NRS) ≥ 4, or change in Children’s Dermatology Life Quality Index (CDLQI)/Infants’ Dermatitis Quality of Life Index (IDQoL) ≥ 6 (full analysis set (FAS) and Investigator’s Global Assessment (IGA) > 1 subgroup). TCS: topical corticosteroids.
In line with these results, the proportions of patients achieving clinically meaningful improvements in both AD signs (EASI-50) and symptoms (≥ 4-point improvement in WSI-NRS) at week 16 were significantly greater for patients receiving dupilumab vs placebo in the IGA > 1 subgroup (35.8% vs 5.6%; p < 0.0001) at week 16 (Fig. S1), with a significant benefit for dupilumab being seen from week 4 onwards. Similar results were observed in the FAS.
In both the IGA > 1 subgroup and the FAS, significantly more patients receiving dupilumab vs placebo achieved EASI-50, EASI-75, and SCORAD-50 (Table SII). In the FAS, significantly more dupilumab-treated patients also achieved EASI-90, with a similar trend in the IGA > 1 subgroup. In addition, in both the IGA > 1 subgroup and FAS, dupilumab vs placebo showed significantly greater mean percentage change from baseline to week 16 in EASI, CDLQI/IDQoL, and WSI-NRS, as well as SCORAD, BSA affected, DFI, Skin Pain NRS, and GISS total. Furthermore, a significantly greater proportion of patients achieved WSI-NRS ≥ 3, WSI-NRS ≥ 4, POEM ≥ 6, CDLQI/IDQoL ≥ 6-point improvement, and CGID “no” or “mild” symptoms at week 16 compared with baseline.
Rescue medication use and adverse events
Fewer patients receiving dupilumab compared with placebo required rescue medication in the IGA > 1 subgroup (26.7% vs 65.3%, respectively) (Table SIII), which was similar to the results observed in the SAS. Moderately potent TCS were the most frequently used rescue medication, and systemic corticosteroids were infrequently used, with no differences between the IGA > 1 subgroup and SAS.
Safety outcomes in patients in the IGA > 1 subgroup were comparable with those in the SAS (Table SIII). Numerically fewer patients receiving dupilumab had ≥ 1 TEAE compared with placebo in the IGA > 1 subgroup (65.0% vs 73.3%, respectively). In both the SAS and the IGA > 1 subgroup, the 3 most common TEAEs were exacerbation of AD, nasopharyngitis, and upper respiratory tract infection (Table SIII). In addition, the only serious TEAEs to occur (exacerbation of AD) were in the placebo groups (Table SIII). One dupilumab-treated patient and 1 placebo-treated patient (the same patients present in the SAS and the IGA > 1 subgroup) discontinued treatment due to a non-serious TEAE (dupilumab group: exacerbation of AD; placebo group: nightmares) (Table SIII).
DISCUSSION
In this subgroup analysis of data from the LIBERTY AD PRESCHOOL part B study, children aged 6 months to 5 years with moderate-to-severe AD who received dupilumab plus low-potency TCS for 16 weeks and whose IGA was > 1 achieved clinically meaningful and statistically significant improvements in the extent and severity of AD lesions, pruritus, and QoL compared with those who received placebo plus TCS. Significant improvement was observed as early as week 2, and progressively increased through the 16-week study duration.
Dupilumab-treated children experienced benefits in objective clinical signs, pruritus, and QoL, whether these parameters were assessed by a composite endpoint or by each instrument individually. Moreover, the magnitude of improvement as measured by EASI, CDLQI, and WSI-NRS in the IGA > 1 population was comparable with that observed in the overall population (22).
In the IGA > 1 subgroup, there were significantly greater improvements at week 16 for dupilumab vs placebo in the other clinician- and patient-reported outcomes including SCORAD, proportion of BSA affected by AD, Skin Pain NRS, POEM, and GISS total. In addition, for patients treated with dupilumab vs placebo, significantly more caregivers reported that their children had “no” or “mild symptoms” at week 16 as assessed by CGID, while the benefit of dupilumab treatment on the QoL of patients’ families was reflected by significantly greater changes from baseline in DFI. The results for the IGA > 1 subgroup were consistent with those previously reported for the overall population.
The safety profile observed in the IGA > 1 subgroup was comparable with the overall population and consistent with the known safety profile of dupilumab observed in older children, adolescents, and adults with moderate to severe AD treated with dupilumab.
The advantages of IGA include simplicity of administration and ease of interpretability. IGA scales have been widely used in AD clinical trials, and the US Food and Drug Administration (FDA) requires IGA ≤ 1 as a primary endpoint for new drug approval trials in AD (13–15). However, the use of IGA has major limitations: it does not account for lesion extent, anatomical location, or symptomatic burden. The fact that dupilumab-treated patients with IGA > 1 experienced improvement in other key clinical outcomes suggests that IGA fails to capture the full spectrum of treatment benefits, especially concerning symptoms and QoL (22). A more holistic approach to evaluating treatment responses, such as the use of a composite endpoint that encompasses clinically relevant and patient-reported outcomes including signs, symptoms, and QoL, may better capture the complex multidimensional nature of AD. Measures that provide a more comprehensive evaluation of treatment effects, including the patient’s own experience, may be more relevant to the decision-making process in real-world clinical practice, and of practical value to prescribing physicians.
Limitations of this analysis include its post hoc nature and the relatively short study duration of 16 weeks, which may not be a sufficient period for assessment of an optimal IGA response. In addition, the minimum clinically meaningful improvement in POEM reported in this analysis has only been validated for children aged 6–11 years, and not yet in younger children.
In summary, the benefits of dupilumab in patients with moderate-to-severe AD are clinically meaningful and statistically significant beyond the stringent IGA ≤ 1 criterion, as demonstrated by multiple validated clinician- and patient-reported outcome measures, such as EASI, CDLQI, pruritus NRS, and POEM.
ACKNOWLEDGEMENTS
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g. FDA, EMA, PMDA), if there is legal authority to share the data and there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
This research was sponsored by Sanofi and Regeneron Pharmaceuticals Inc. ClinicalTrials.gov Identifier: NCT03346434. The study sponsors participated in the study design; collection, analysis, and interpretation of the data; writing of the report; and the decision to submit the article for publication. Medical writing/ editorial assistance were provided by Liselotte van Delden of Excerpta Medica, and was funded by Sanofi and Regeneron Pharmaceuticals Inc., according to the Good Publication Practice Guidelines.
REFERENCES
- Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol 2021; 126: 417–428.e2.
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel) 2019; 6: 133.
- Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr 2019; 173: e190025.
- Barbarot S, Silverberg JI, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. The family impact of atopic dermatitis in the pediatric population: results from an international cross-sectional study. J Pediatr 2022; 246: 220–226.e5.
- Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71: 327–349.
- Macdonald LE, Karow M, Stevens S, Auerbach W, Poueymirou WT, Yasenchak J, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A 2014; 111: 5147–5152.
- Murphy AJ, Macdonald LE, Stevens S, Karow M, Dore AT, Pobursky K, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A 2014; 111: 5153–5158.
- Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014; 371: 130–139.
- Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–2348.
- Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287–2303.
- Simpson EL, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156: 44–56.
- Paller AS, Simpson EL, Siegfried EC, Cork MJ, Wollenberg A, Arkwright PD, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2022; 400: 908–919.
- Paller AS, Siegfried EC, Thaçi D, Wollenberg A, Cork MJ, Arkwright PD, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol 2020; 83: 1282–1293.
- Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol 2016; 74: 288–294.
- Chalmers JR, Schmitt J, Apfelbacher C, Dohil M, Eichenfield LF, Simpson EL, et al. Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 2014; 171: 1318–1325.
- Silverberg JI, Simpson EL, Ardeleanu M, Thaçi D, Barbarot S, Bagel J, et al. Dupilumab provides important clinical benefits to patients with atopic dermatitis who do not achieve clear or almost clear skin according to the Investigator’s Global Assessment: a pooled analysis of data from two phase III trials. Br J Dermatol 2019; 181: 80–87.
- Paller AS, Bansal A, Simpson EL, Boguniewicz M, Blauvelt A, Siegfried EC, et al. Clinically meaningful responses to dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: post-hoc analyses from a randomized clinical trial. Am J Clin Dermatol 2020; 21: 119–131.
- Siegfried EC, Cork MJ, Katoh N, Zhang H, Chuang C-C, Thomas RB, et al. Dupilumab provides clinically meaningful responses in children aged 6–11 years with severe atopic dermatitis: post-hoc analysis results from a phase III trial. Am J Clin Dermatol 2023; 24: 787–798.
- Paller A, Siegfried E, Marron SE, Clark M, DiBenedetti D, Nelson L, et al. Development and validation of a caregiver-reported numeric rating scale for measuring pruritus in children aged 6 months to < 6 years with atopic dermatitis. [abstract]. J Invest Dermatol 2022 Aug; 142:S25. doi: 10.1016/j.jid.2022.05.154.
- Simpson EL, de Bruin-Weller M, Bansal A, Chen Z, Nelson L, Whalley D, et al. Definition of clinically meaningful within-patient changes in POEM and CDLQI in children 6 to 11 years of age with severe atopic dermatitis. Dermatol Ther (Heidelb) 2021; 11: 1415–1422.
- Paller AS, Marron SE, Whalley D, Qin S, Chao J, Bansal A, et al. Clinically meaningful within-patient change threshold for the Children’s Dermatology Life Quality Index and Infants’ Dermatitis Quality of Life Index instruments in patients aged 6 months to < 6 years with atopic dermatitis [abstract]. Poster presented at the 2022 European Academy of Dermatology and Venereology (EADV) Annual Meeting; Sept 7–10, 2022; Milan, Italy.
- De Bruin-Weller M, Thaçi D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol 2018; 178: 1083–1101.