Targeted therapy with a BRAF inhibitor and a MEK inhibitor (BRAFi/MEKi) is a well-established treatment option for patients with BRAF-mutated advanced stage melanoma (1). In disease progression, sequencing between immune checkpoint inhibitors (ICI) and BRAFi/MEKi is a common approach (1, 2). BRAFi/MEKi treatment is generally well tolerated and usually causes only moderate side-effects (1), but serious adverse events, including severe skin toxicity, have been described (1, 3–5). Recently, the question of efficacy, tolerability and possible toxicity of sequential therapy has been raised (5, 6). We report here the first case of concomitant development of Stevens-Johnson syndrome (SJS) and isolated severe anaemia in a patient undergoing treatment with vemurafenib plus cobimetinib following pembrolizumab therapy for advanced stage BRAF-mutated melanoma.
CASE REPORT
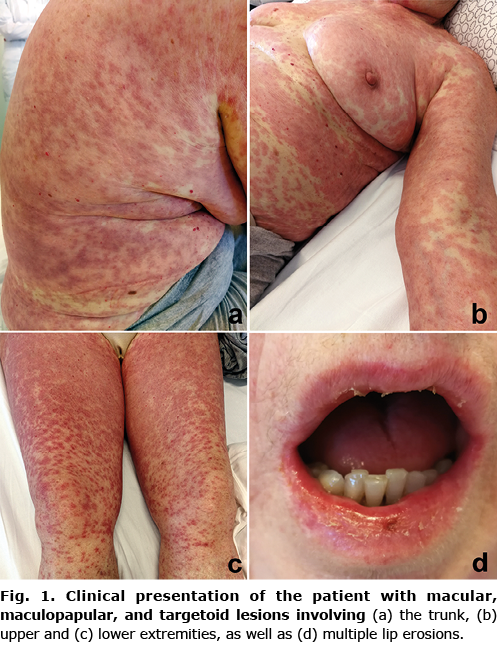
A 68-year-old woman with a history of metastatic melanoma presented with progressive maculopapular rash of 5 days’ duration, accompanied by fever up to 38.2°C, chills, hypotension, tachycardia, and malaise, in addition to itching and burning of the skin. The patient was diagnosed with melanoma of the right arm (Breslow thickness 2.2 mm, pathological stage pT3b) in 2016 with negative sentinel lymph node biopsy of the right axillary region. Three years later, positron emission tomography-computed tomography (PET-CT) confirmed extensive disease with numerous metastatic lesions. Molecular examination of the primary lesion revealed the presence of a BRAF V600E mutation. In May 2019, immunotherapy with pembrolizumab was initiated and well tolerated. At the end of the 4th month of treatment, the patient developed deep vein thrombosis in the left leg, which was treated successfully with low molecular weight heparin and subsequently with rivaroxaban. PET-CT at re-evaluation after 8 months showed progression of the disease with the appearance of new metastatic lesions and progression of the older lesions (Fig. S1a). The patient was switched to targeted therapy with vemurafenib and cobimetinib, but within 8 days she began to develop a generalized rash and fever. Skin examination revealed a confluent maculopapular rash distributed predominantly on the trunk and upper extremities, with multiple atypical targetoid lesions (Fig. 1a, b), associated with several painful erosions in the mouth and conjunctivitis. Routine laboratory tests revealed severe anaemia with red blood cells (RBCs) 2.81 × 1012/l, haemoglobin (Hgb) 57 g/l, haematocrit (Hct) 0.200 l/l, mean corpuscular volume (MCV) 71.1 fl, mean corpuscular haemoglobin (MCH) 20.4 pg, iron (Fe) 3 µmol/l, ferritin <8 µg/l, leukocytosis 23.6 × 109/l, neutrophilia 21.95 × 109/l, elevated C-reactive protein (CRP) to 131.1 mg/l, signs of acute renal failure, but no alteration in liver enzymes. Vemurafenib and cobimetinib were immediately discontinued and parenteral methylprednisolone (1.5 mg/kg/day), chloropyramine and pantoprazole were administered along with supportive measures. Although 2 doses of RBCs transfusion were given after admission, the anaemia continued to progress the next day (RBCs 2.51 × 1012/l, Hgb 51 g/l, Hct 0.171 l/l, requiring a total of 6 doses of RBCs to maintain Hgb level. In the following days, skin and mucosal lesions developed into full-blown SJS with rash spreading to the lower extremities and face, along with the appearance of stomatitis and cheilitis, characterized by multiple crusted lip erosions (Fig. 1c, d) and difficulty chewing. The patient also reported painful swallowing, indicating the additional affect of the pharyngeal mucosa. Laboratory results showed rapid improvement in renal function, a decrease in inflammatory markers, and normalization of leukocyte counts. Over the course of 2 weeks, the rash gradually resolved, and the anaemia was adequately corrected (E 3.95 × 1012/l, Hgb 100 g/l, Hct 0.304 l/l), allowing methylprednisolone taper. A detailed workup ruled out haemolytic anaemia and recent significant gastrointestinal, urogenital, or other causes of manifest bleeding, although stool examination was initially positive for occult bleeding. The patient was switched to the alternative BRAFi/MEKi combination, namely dabrafenib (2 × 150 mg/day) plus trametinib (2 mg/day) 2 weeks after hospital discharge, while continuing methylprednisolone taper. During further 1-year follow-up, there was no evidence of relapse or progression of anaemia and/or rash. Computed tomography (CT) scan showed signs of marked regression of metastatic disease (Fig. S1b–d), while laboratory values remained within reference intervals.
DISCUSSION
Adverse reactions are frequently observed with all 3 BRAFi/MEKi combinations, but most commonly associated with vemurafenib plus cobimetinib therapy (1, 3–6). Although most cutaneous adverse effects are mild, severe and potentially life-threatening reactions leading to permanent discontinuation, interruption or dose reduction of therapy have also been reported, including erythema multiforme, SJS, toxic epidermal necrolysis (TEN), drug rash with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) (3, 5, 7–9). Sequential treatment with BRAFi/MEKi in patients with melanoma who have previously received ICI targeting programmed cell death protein 1 (PD-1), could result in a different safety profile, increased systemic and skin toxicity, with more than 50% of patients developing grade 3–5 adverse events (5, 6, 10, 11). Recent literature data on severe skin reactions due to sequential therapy suggest a more frequent occurrence of SJS/TEN and DRESS, while AGEP and the isolated form of SJS are rare, with vemurafenib being the most common culprit drug (3, 8, 9, 11–13). The rapid onset of systemic hypersensitivity and early skin reaction after initiation of BRAFi/MEKi following ICI therapy could be due to persistent immune changes exacerbating the severity of the drug reaction caused by targeted therapy (5, 6, 11), although in the current case we assumed that 4 weeks after discontinuation of pembrolizumab was a sufficient washout period. In addition to skin toxicity, the current patient abruptly developed severe anaemia with a rapid drop in normal Hgb values to 51 g/L after the introduction of vemurafenib plus cobimetinib, suggesting acute bleeding. Anaemia is the most common haematological side-effect of targeted therapy noted in clinical trials (1). It occurs in 15% of patients, but severe anaemia in only 1.6% of patients receiving vemurafenib plus cobimetinib (4). Cytopaenias were frequently observed in patients receiving BRAFi/MEKi after anti-PD-1 therapy (13%) (6). Patients on BRAFi/MEKi therapy may experience bleeding manifested as melena, haematuria, haemoptysis, haematemesis, vaginal bleeding, or bleeding within liver or bone metastases. This is likely due to a rapid response to treatment leading to tumour necrosis (14), the latter most likely being the cause of severe anaemia in the current patient. After ruling out actual acute bleeding, laboratory findings indicated the presence of sideropaenic anaemia, possibly due to nonmanifest bleeding within metastases (14) or previous pembrolizumab therapy (15). Systemic immune activation by ICI could also affect the coagulation-fibrinolysis system, leading to hypercoagulopathy and thromboembolic events causing bleeding complications, such as purpura, intra-tumoural haemorrhage, bronchial or gastrointestinal haemorrhage (15). The severity and slow recovery of anaemia despite blood transfusions could be related to robust systemic inflammation associated with SJS or pre-existing sideropenia in chronic disease. There was no relapse of anaemia after switching to a different BRAFi/MEKi combination, suggesting that isolated cytopaenia could be attributed to a broad spectrum of toxicity induced by vemurafenib plus cobimetinib. Consistent with previous reports (8, 9, 13), switching to dabrafenib appeared to be both a successful and safe alternative for the treatment of metastatic melanoma in our patient.
This case highlights the possibility of simultaneous development of a severe skin reaction and severe anaemia due to sequential treatment with vemurafenib plus cobimetinib following checkpoint blockade in patients with BRAF-mutated advanced melanoma and the importance of close monitoring.
ACKNOWLEDGEMENTS
The authors thank the patient for granting permission to publish this information. We acknowledge the health personnel of the Dermatovenereology Department, Clinical Hospital Center of Rijeka who took part in the management of this patient. Images of PET-CT and CT scans were obtained by the courtesy of M. Ciglar Hlašć, MD, nuclear medicine specialist.
The authors have no conflicts of interest to declare.
REFERENCES
- Heinzerling L, Eigentler TK, Fluck M, Hassel JC, Heller-Schenck D, Leipe J, et al. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open 2019; 4: e000491.
- O’Reilly A, Larkin J. Checkpoint inhibitors in advanced melanoma: effect on the field of immunotherapy. Expert Rev Anticancer Ther 2017; 17: 647–655.
- Torres-Navarro I, de Unamuno-Bustos B, Botella-Estrada R. Systematic review of BRAF/MEK inhibitors-induced severe cutaneous adverse reactions (SCARs). J Eur Acad Dermatol Venereol 2021; 35: 607–614.
- Dréno B, Ribas A, Larkin J, Ascierto PA, Hauschild A, Thomas L, et al. Incidence, course, and management of toxicities associated with cobimetinib in combination with vemurafenib in the coBRIM study. Ann Oncol 2017; 28: 1137–1144.
- Lamiaux M, Scalbert C, Lepesant P, Desmedt E, Templier C, Dziwniel V, et al. Severe skin toxicity with organ damage under the combination of targeted therapy following immunotherapy in metastatic melanoma. Melanoma Res 2018; 28: 451–457.
- Saab KR, Mooradian MJ, Wang DY, Chon J, Xia CY, Bialczak A, et al. Tolerance and efficacy of BRAF plus MEK inhibition in patients with melanoma who previously have received programmed cell death protein 1-based therapy. Cancer 2019; 125: 884–891.
- Gey A, Milpied B, Dutriaux C, Mateus C, Robert C, Perro G, et al. Severe cutaneous adverse reaction associated with vemurafenib: DRESS, AGEP or overlap reaction? J Eur Acad Dermatol Venereol 2016; 30: 178–179.
- Tahseen AI, Patel NB. Successful dabrafenib transition after vemurafenib-induced toxic epidermal necrolysis in a patient with metastatic melanoma. JAAD Case Rep 2018; 4: 930–933.
- Pinard C, Mignard C, Samain A, Duval-Modeste A-B, Joly P. Successful use of dabrafenib after the occurrence of drug rash with eosinophilia and systemic symptoms (DRESS) induced by vemurafenib. JAAD Case Rep 2017; 3: 532–533.
- Broman KK, Dossett LA, Sun J, Eroglu Z, Zager JS. Update on BRAF and MEK inhibition for treatment of melanoma in metastatic, unresectable, and adjuvant settings. Expert Opin Drug Saf 2019; 18: 381–392.
- Naqash AR, File DM, Ziemer CM, Whang YE, Landman P, Googe PB, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. A single-institutional case-series. J Immunother Cancer 2019; 7: 4.
- Poduje S, Brozić JM, Prkačin I, Delaš Aždajić M, Goren A. Vemurafenib and cobimetinib-induced toxic epidermal necrolysis in a patient with metastatic melanoma. Dermatol Ther 2020; 33: e13174.
- Maximova N, Maestro A, Zanon D, Marcuzzi A. Rapid recovery of post nivolumab vemurafenib-induced Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) syndrome after tocilizumab and infliximab administration. J Immunother Cancer 2020; 8: e000388.
- Loyson T, Werbrouck E, Punie K, Bonne L, Vandecaveye V, Bechter O. Hemorrhage of liver and bone metastases as a result of rapid response to dual BRAF/MEK inhibition in metastatic melanoma: a case report. Melanoma Res 2018; 28: 147–150.
- Sato R, Imamura K, Sakata S, Ikeda T, Horio Y, Iyama S, et al. Disorder of coagulation-fibrinolysis system: an emerging toxicity of anti-PD-1/PD-L1 monoclonal antibodies. J Clin Med 2019; 8: 762.