Graft-versus-host disease (GVHD) is a common complication of haematopoietic stem cell transplantation. This study examined the cutaneous microbiome in relation to the pathogenesis of cutaneous GVHD. Bacterial swabs were taken from several sites on 12 patients with cutaneous GVHD. Microbiotas were characterized by sequencing 16S rRNA bacterial genes on the MiSeq platform. Microbiome diversity in patients with cutaneous GVHD was reduced compared with healthy controls. GVHD was related to an increased abundance of Firmicutes and a reduction in Actinobacteria, especially in lesions. Non-parametric multivariate analysis of variance revealed that the skin microbial community disorders in patients with GVHD correlated with several clinical features of cutaneous GVHD. This study indicates that changes in the cutaneous microbiota in lesions could play a key role in the pathogenesis of cutaneous GVHD. Further studies are needed to explore the mechanistic relevance of these microbial dynamics, which may provide new clues to therapeutic interventions.
Key words: skin microbiome; graft versus host disease; 16S rRNA; Staphylococcus; firmicutes.
Accepted Aug 13, 2020; Epub ahead of print Aug 18, 2020
Acta Derm Venereol 2021; 101: adv00374.
doi: 10.2340/00015555-3613
Corr: Jianzhong Zhang, Department of Dermatology, Peking University People’s Hospital, No. 11 South Street of Xizhimen, Xicheng District, Beijing 100044, China. E-mail: rmdrzjz@126.com
SIGNIFICANCE
Graft-versus-host disease is a severe adverse effect of haematopoietic stem cell transplantation and has a high rate of mortality. The skin is one of the major organs affected. This study represents the first comprehensive analysis of the skin microbiome in patients with graft-versus-host disease. The results indicate that graft-versus-host disease skin exhibits a less diverse skin microbiome compared with healthy skin, with a greater overall abundance of Staphylococcus. The abnormal skin microbiome in graft-versus-host disease dysbiosis may help in our understanding of the pathogenesis of cutaneous graft-versus-host disease.
INTRODUCTION
The skin microbiota cohabits the skin surface and may play a positive or negative role in the immune system (1). Skin microorganisms can influence host cells; for example, by moderating the production of endogenous antimicrobial peptides, and promoting host immunity by contributing to the innate and adaptive defence system (2–4). Skin microbial dysbiosis has been correlated with various skin diseases, such as atopic dermatitis (5), acne vulgaris (6), psoriasis (7) and vitiligo (8). The skin microbiota may play a role in autoimmune diseases, including bullous pemphigoid, systemic lupus erythematosus and dermatomyositis (9, 10).
Graft-versus-host disease (GVHD) is a severe adverse event with a high rate of mortality resulting from haematopoietic stem cell transplantation (HSCT) (11). GVHD is initiated when donor CD4+ or CD8+ T cells become activated and infiltrate into multiple recipient tissues, namely the skin, gut, liver and lung, where they recognize major histocompatibility complex (MHC) class I or class II molecules in the recipient (12). Although recent work has advanced our knowledge of GVHD biology, it also underscores the complexity of mechanisms capable of initiating and propagating GVHD (13). Specifically, it remains unclear as to why GVHD exhibits such tissue specificity for the gut, skin, liver and lung, and why it occurs in only a proportion of patients. The fact that these tissues targeted by GVHD also sustain the highest bacterial loads in the body is unlikely to be coincidental; indeed, there is evidence regarding the effect of gut microbiota on development of GVHD. The gut microbiota not only modulates gastrointestinal immune homeostasis, it also contributes to the maintenance of epithelial cells (14–16). Microbiota shifts, from dominance of Clostridial to dominance of Lactobacillales and Enterobacteriales (15, 17, 18), can be found in patients with GVHD. Since Clostridial species can prevent inflammation, by upregulating regulatory T cells in the intestines, there is speculation that GVHD may reduce anti-inflammatory cell frequency by reducing the abundance of Clostridiales (19). Skin-restricted commensal colonization that accelerates skin graft rejection has been demonstrated in a mouse model (20). However, the characteristics and variation in skin microbiome in the lesions and non-lesional skins of patients with GVHD are little known.
Previously, our understanding of the relevance of microorganisms in GVHD were hindered by the limitations of culture-dependent methods. Currently, the most effective approach relies on amplifying the phylogenetically informative 16S ribosomal RNA gene of bacteria (16S rRNA) (21, 22).
This study characterized and compared the skin microbiome at different locations between patients with cutaneous GVHD and healthy controls.
MATERIALS AND METHODS
Study populations
This study was carried out based on the principles of the Declaration of Helsinki and was approved by the ethics committee of Peking University People’s Hospital. Twelve patients with cutaneous GVHD were sampled between April 2018 and November 2019 in the dermatology department at Peking University People’s Hospital. All 12 patients (7 males and 5 females; 17–49 years old) received HSCT and satisfied the National Institutes of Health (NIH) criteria for GVHD (16338616). Involved skin lesion areas in all the patients were less than 50% body surface area (BSA). Skin biopsies were performed in 4 patients. Clinical features of the patients with cutaneous GVHD were collected. Fifteen sex- and age-matched subjects were used as controls. Written informed consent was obtained from all participants for skin sample collection and analysis. Participants with a history of cancer, autoimmune disease, bloodstream infection or those receiving antibiotic therapy for at least 4 weeks were excluded.
Sample collection
Skin samples were taken by swabbing a 2×2 cm area of the selected skin sites, under near-sterile conditions with sterile swabs immersed in sterile NaCl (0.15 M) with 0.1% Tween 20 (Fisher Scientific, Fair Lawn, NJ, USA). Sampling was carried out by the same investigator, who wore disposable gloves and a mask.
Samples were obtained from typical lesions on flexor side of the forearm (designed as GVHD lesions). The clinically unaffected skin adjacent to the lesions was sampled (designed GVHD non-lesions). Sampling was also performed on several unaffected sites, including the forehead and back. Sampling was also performed in healthy individuals. All participants were instructed to avoid using topical medications and emollients for one week, and to avoid bathing for at least 24 h prior to sampling. Skin samples were stored rapidly at –80°C for preparation.
DNA extraction and sequencing
Bacterial DNA was extracted from the swabs using DNeasy Tissue Kit (Qiagen, Chatsworth, CA, USA). Micro-centrifuge filter (MW threshold 30,000 Daltons, Amicon, Bedford, MA, USA) was used for filtration to avoid contamination. Skin microbiome libraries for sequencing were prepared as described previously (23). V3-V4 16S rDNA was amplified using custom-made primers (forward primer (5′-ACT CCT ACG GGA GGC AGC AG-3′); reverse primer (5′-GGA CTA CHV GGG TWT CTA AT-3′)). Amplified DNA was quantified using a PicoGreen assay (Invitrogen) so that equal amounts of DNA from each sample could be pooled and cleaned using the UltraClean PCR Clean-up protocol (Qiagen).
Sequencing was carried out at Huada Medical Laboratory (Wuhan, China). DNA fragments shorter (< 300 nt) or longer (> 1,000 nt) than the expected amplicon target site were removed. The qualified libraries were sequenced pair end on the MiSeq platform (Illumina Inc., CA, USA), using Trimmomatic (24) and FLASH (25) as described previously.
Raw sequencing data were processed using USEARCH, as described previously (26). To perform taxonomic classification, MOTHUR version V.1.39.5 (https://www.mothur.org) were used.
Statistical analyses
Alpha diversity (Sobs, Chao1) and beta diversity (Jaccard Index) were calculated using Mothur and Anosim. MetaStats 2.0 package (27) and Krona (https://github.com/marbl/Krona/wiki) were used to measure metagenomics constitutions at every phylogenetic level, from kingdom to species, to identify taxa that were statistically enriched or reduced in one situation compared with the other. To analyse the correlation of skin microbiome with the clinical features of cutaneous GVHD, non-parametric multivariate analysis of variance (Adonis) was applied. The following disease-related variables were used in Adonis: age, sex of patient, primary disease, sex of donor, prophylactic medication, time of skin rejection onset, itching, dry skin, extracutaneous involvement and type of cutaneous GVHD. Wilcox-test was used between 2 sample groups and Kruskal–Wallis test was performed for multiple sample groups. p < 0.05 was considered statistically significant for all comparisons.
RESULTS
Demographic of participants and taxonomic distribution
Six patients with atopic dermatitis (AD)-like GVHD and 6 with lichen planus (LP)-like GVHD were included (5 women and 7 men, age range 17–49 years). None of the patients underwent total body irradiation or donor lymphocytic infusions. The patients with atopic dermatitis (AD)-like GVHD had no previous atopy history. Primary diseases included acute myeloid leukaemia (8 patients) and acute lymphoblastic leukaemia (4 patients) (Table I). All 12 donors were first-degree family members.
The final sequence data contained 6,331,157 16S rRNA sequences and 2,708 species-level operational taxonomic units (OTU). The following distribution of unique named taxa are classified: 33 phyla, 50 classes, 98 orders, 144 families, 584 genera and 779 species. The most abundant genus was consistently Staphylococcus: AD-like GVHD (42%), LP-like GVHD (31%).
Abundance of bacterial phyla
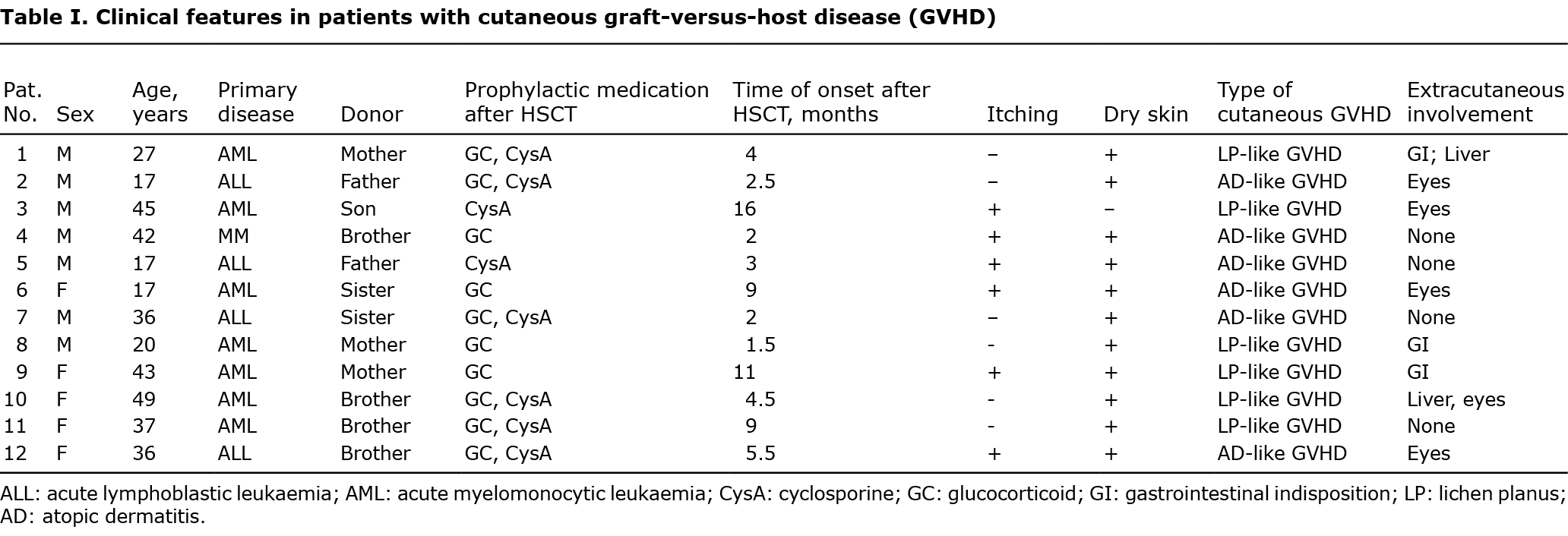
Location- and disease-dependent variances were identified according to the abundance of bacterial phyla. Firmicutes, Proteobacteria and Actinobacteria dominate the cutaneous microbial communities in all sampling locations, which is similar to previous studies of human skin microbiome at the phylum level (28). Increased Firmicutes and decreased Actinobacteria were found on the back of patients with GVHD (Fig. 1a). The proportion of the Actinobacteria and Firmicutes abundance were both decreased on the forehead of patients with GVHD (Fig. 1a). The altered abundance of Proteobacteria, Firmicutes, Deferribacteres and Parcubacteria were significantly different among lesions, non-lesional skin and healthy controls (Fig. 1b). The most striking shift occurred for the Firmicutes phylum, in going from healthy to lesional skin of patients with GVHD. Thus, a decrease in the Actinobacteria to Firmicutes-ratio was already found on the back skin of patients with GVHD (Fig. 1c, p = 0.21), which was further marked in lesions (Fig. 1c, p = 0.001).
Abundance of bacterial genera
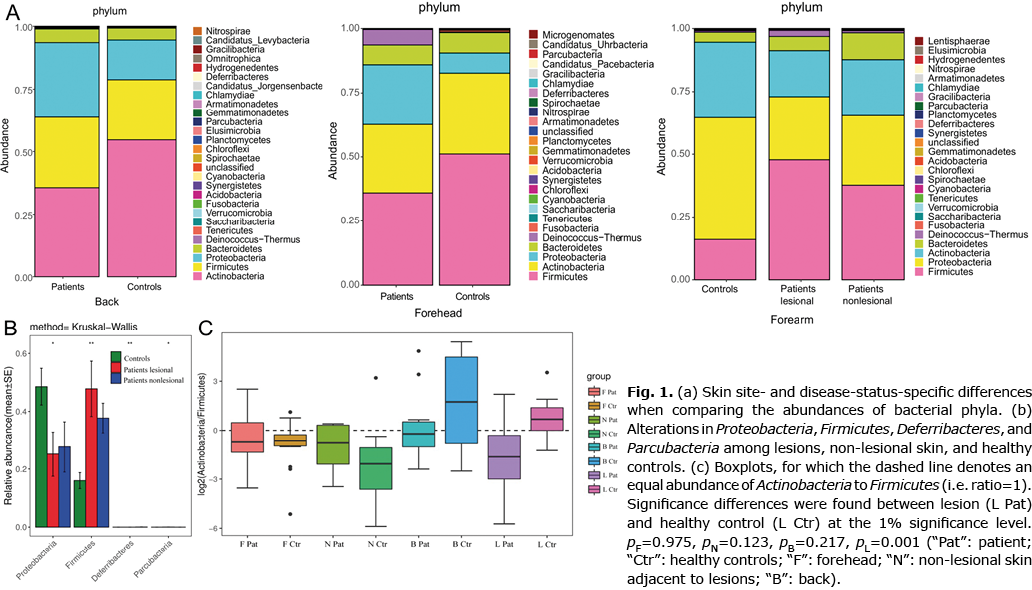
In terms of genera-level, the most dramatic variance was the dominance of Propionibacteria on healthy back skin and the significant decrease in these in patients (Fig. 2). A gradual increased tendency of Staphylococcus, from healthy individuals to GVHD non-lesional skin and to lesional skin was found. On the other hand, when comparing matched sites, Pseudomonas and Propionbacteria showed a dramatic reduction in both GVHD lesions and non-lesional skin (Fig. 2). The proportion of Staphylococcus was markedly increased in GVHD lesions (36% vs 9%) and, to a lesser extent, in unaffected skin (forehead: 29% vs 26%; back: 15% vs 6%; adjacent skin: 17% vs 7%) using Krona, suggesting that a shift in the abundance of Staphylococcus was already observed in non-lesional skin of patients, which was much more obvious in lesions.
Alpha diversity
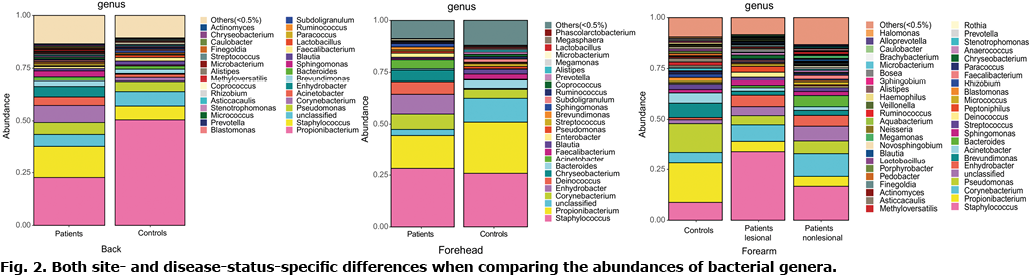
Bacterial diversity (alpha diversity) of the microbiome was investigated using Sobs and Chao index (29), which reflect species-richness. To determine whether the diversity of the skin microbiome is altered in cutaneous GVHD the microbiome in lesions of GVHD, non-lesional adjacent skin and healthy skin were first identified using 16S rRNA sequencing. Species accumulation curves implied that the sequencing data met analysis conditions. Alpha diversity analysed by Sobs and Chao1 displayed a large reduction on a non-lesional forehead site in patients with GVHD compared with healthy controls (Fig. 3a). Alpha diversity (Sobs and Chao1) was decreased on the back skin of patients with cutaneous GVHD compared with healthy controls (Fig. 3b, p > 0.05). There was no significant difference in alpha diversity between GVHD lesions and normal skin. Interestingly, less community diversity was found in AD-like GVHD lesions compared with non-lesional sites (Fig. 3c). However, the community diversity between lesions and non-lesional skin in patients with LP-like GVHD was similar (Fig. 3d). This implies less microbial diversity in AD-like GVHD lesions, compared with non-lesional skin.
Beta diversity
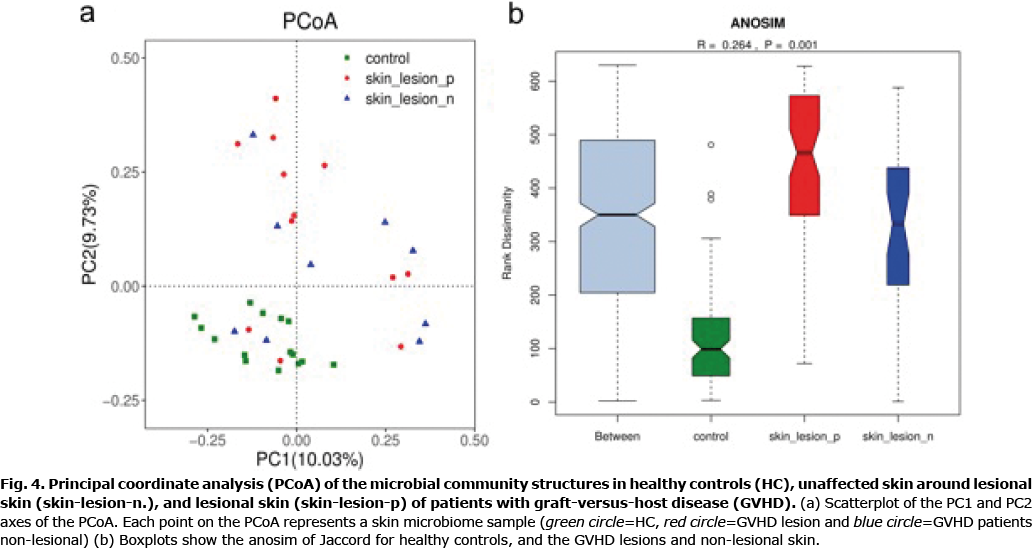
The overall microbiome composition (beta diversity) was analysed and visualized by principal coordinates analysis (PCoA) (30). Beta diversity of the cutaneous GVHD microbiota and healthy controls was measured. Principal coordinates (PC1 and PC2) represented 10.03% and 9.73% of alterations, respectively. A distinct detachment between microbial communities was observed in both axes (Fig. 4a), suggesting that the composition of microbial communities from GVHD lesions and healthy controls showed significant differences. Considering the marked clusters associated with various cutaneous conditions, the microbial communities in either GVHD non-lesional skin or GVHD lesions were more scattered in the PCoA than those from healthy controls. This implied that the skin microbial composition from patients with GVHD were more heterogeneous in either lesions or non-lesional skin. The microbial community dispersion of every group was further measured using Jaccard (Fig. 4b, R = 0.264, p = 0.001). These results indicated that the non-lesional skin might be a transition state between lesions and controls. In addition, beta diversity was significantly different between patients and controls in lesional sites (p = 0.001) and non-lesional sites (p = 0.001). The lesional site has more increasing trend than the non-lesional site in patients with GVHD, although the difference is less significant (p = 0.923). These results imply that skin microbiome constitution in patients with GVHD was absolutely different from healthy controls. Cutaneous GVHD presented more variety of skin microbiome in either GVHD lesions or GVHD non-lesional skin.
Association of skin microbiome with clinical features of cutaneous graft-versus-host disease
At the OTU level, non-parametric multivariate analysis of variance (Adonis) was used to investigate the association between skin microbiome and clinical features of patients with cutaneous GVHD. Age, prophylactic medication, primary disease, sex of donor, itching, prophylactic medication, extracutaneous involvement, time of skin rejection onset and type of cutaneous GVHD (AD-like GVHD or LP-like GVHD) correlated with the microbial diversity of GVHD lesions. In contrast, no relationship was found between the sex of the patient and dry skin, and the diversity of the skin microbiome in GVHD lesions.
DISCUSSION
Commensal microbiome contributes to biological processes, such as immunity and metabolism, and host-microbe interactions have been found to be correlated with human physiology (31, 32). Consisting of commensal and pathogenic bacteria, the skin microbiome may be involved in epithelial innate immune responses. Skin microbiome composition is affected by skin locations and remains generally stable over time. Skin microbiome has been found to have an important role in the initiation and maintenance of inflammatory skin diseases, and its connection with the immune system have been found in multiple diseases, including atopic dermatitis, psoriasis, allergic contact dermatitis, autoimmune blistering disorders and systemic lupus erythematosus (9, 10).
The gut microbiome has been reported to play an important role in the development of GVHD. Decreased microbiota diversity is an independent risk factor (15). Taur et al. reported that low microbial diversity was linked to administration of antibiotics and myeloablative conditioning (33). Increasing studies have shown that both the leaky bacterial product and the bacteria translocation had impact on the immune system, not only in the intestine, but also in the whole organism (34). Recent clinical literature suggests that a host’s graft-rejection process may be associated with shifts in the bacterial composition of the skin (35, 36). Patients presenting cutaneous GVHD are generally found to have dryer skin and an impaired skin barrier (37), implying that their skin microbiota may have been altered. In the mouse model, a single commensal skin species, Staphylococcus epidermidis, was found to be capable of accelerating the rejection of skin grafts (20).
This study reports the first comprehensive analysis of the skin microbiome in patients with GVHD. Its alpha diversity was diminished at numerous unaffected sites in patients with GVHD, compared with healthy controls, suggesting that the hosts’ disease state may affect microbial diversity, in that abnormally-activated immune responses may explain reduced alpha diversity in GVHD skin. Furthermore, more diversity was lost at the lesional site than the unaffected site in patients with AD-like GVHD, but not in patients with LP-like GVHD. Previous studies have shown that, for AD-like GVHD and LP-like GVHD conditions, both patients had elevated Th2 cells and impaired skin barrier, whereas the counts of eosinophils, Th17 cells, and Treg cells only increased under the former condition (38, 39). According to recent work, intestinal microbiota can induce GVHD by influencing the Treg/Th17 balance (37, 40). There was no significant difference in alpha diversity between cutaneous GVHD lesions and healthy controls. Similarly, Miodovnik et al. (9) reported that alpha diversity was not strictly affected by disease state in patients with bullous pemphigoid.
The difference in beta diversity between patients and healthy controls in lesions and non-lesional skin might imply different skin microbiome between patients with cutaneous GVHD and healthy controls. The Adonis analysis showed that age, primary disease, sex of donor, prophylactic medication, time of skin rejection onset, itching, extracutaneous involvement and type of cutaneous GVHD (AD-like GVHD or LP-like GVHD) were associated with the microbial diversity of GVHD lesions. The different cutaneous microbiota for patients with GVHD and controls, which could theoretically be due to the reasons mentioned. In addition, we found that beta diversity had a more increasing trend in lesions than in non-lesional skin within GVHD patients, although the difference is not very significant. This suggests that the GVHD lesions may directly influence beta diversity. This speculation calls for a larger scale study.
In this study, Firmicutes, Proteobacteria and Actinobacteria are the phyla dominating in the skin microbiome in lesion, non-lesional GVHD skin and healthy skin, which is similar to previous studies (28, 41). Spirochaetae and Parcubacteria were significantly different from patients with cutaneous GVHD and healthy controls, which may discriminate GVHD lesions from healthy skin. A major genus belonging to Firmicutes is Staphylococcus. PICRUSt analysis revealed that the S. aureus infection pathway significantly enriched and strong correlated with genus Staphylococcus in SLE patients (10). Listeria monocytogenes, S. aureus, and S. epidermidis can increase alloreactivity, thereby accelerating skin allograft rejection and preventing the induction of transplantation tolerance by co-stimulation-blocking agents (42, 43), and Acinetobacter species-induced protective immune responses against allergic sensitization and inflammation (44). A decreasing tendency in the Actinobacteria to Firmicutes-ratio was obvious in GVHD lesions. The abnormal skin microbiome in GVHD dysbiosis may present some clues for understanding the pathogenesis of cutaneous GVHD. Whether Staphylococcus plays the role as pathogenic bacteria in the mechanisms of GVHD remains unclear. Future studies should explore specific microorganisms and their roles in the pathogenesis of GVHD.
In conclusion, this study investigated the entire skin microbiome in GVHD. The results indicate that GVHD skin exhibits a less diverse skin microbiome compared with those in healthy skin, with a more overall abundance of Staphylococcus.
The authors have no conflicts of interest to declare.
REFERENCES
- Abdallah F, Mijouin L, Pichon C. Skin Immune landscape: inside and outside the organism. Mediators Inflamm 2017; 2017: 5095293.
- Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol 2011; 131: 1974–1980.
- Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 2009; 15: 1377–1382.
- Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 2010; 130: 2211–2221.
- Clausen ML, Agner T, Lilje B, Edslev SM, Johannesen TB, Andersen PS. Association of disease severity with skin microbiome and filaggrin gene mutations in adult atopic dermatitis. JAMA Dermatol 2018; 154: 293–300.
- Dagnelie MA, Corvec S, Saint-Jean M, Bourdes V, Nguyen JM, Khammari A, et al. Decrease in diversity of propionibacterium acnes phylotypes in patients with severe acne on the back. Acta Derm Venereol 2018; 98: 262–267.
- Langan EA, Kunstner A, Miodovnik M, Zillikens D, Thaci D, Baines JF, et al. Combined culture and metagenomic analyses reveal significant shifts in the composition of the cutaneous microbiome in psoriasis. Br J Dermatol 2019; 181: 1254–1264.
- Ganju P, Nagpal S, Mohammed MH, Nishal Kumar P, Pandey R, Natarajan VT, et al. Microbial community profiling shows dysbiosis in the lesional skin of vitiligo subjects. Sci Rep 2016; 6; 18761.
- Miodovnik M, Kunstner A, Langan EA, Zillikens D, Glaser R, Sprecher E, et al. A distinct cutaneous microbiota profile in autoimmune bullous disease patients. Exp Dermatol 2017; 26: 1221–1227.
- Huang C, Yi X, Long H, Zhang G, Wu H, Zhao M, et al. Disordered cutaneous microbiota in systemic lupus erythematosus. J Autoimmun 2019; 108: 102391.
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol 2007; 7: 340–352.
- Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol 2001; 29; 259–277.
- Paczesny S, Hanauer D, Sun Y, Reddy P. New perspectives on the biology of acute GVHD. Bone Marrow Transplant 2010; 45: 1–11.
- Noor F, Kaysen A, Wilmes P, Schneider JG. The gut microbiota and hematopoietic stem cell transplantation: challenges and potentials. J Innate Immun 2019; 11: 405–415.
- Shono Y, Docampo MD, Peled JU, Perobelli SM, Jenq RR. Intestinal microbiota-related effects on graft-versus-host disease. Int J Hematol 2015; 101: 428–437.
- Murphy S, Nguyen VH. Role of gut microbiota in graft-versus-host disease. Leuk Lymphoma 2011; 52: 1844–1856.
- Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 2014; 20: 640–645.
- Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 2012; 209: 903–911.
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500: 232–236.
- Lei YM, Sepulveda M, Chen L, Wang Y, Pirozzolo I, Theriault B, et al. Skin-restricted commensal colonization accelerates skin graft rejection. JCI Insight 2019; 5: e127569.
- Robinson CJ, Bohannan BJ, Young VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev 2010; 74: 453–476.
- Kong HH. Skin microbiome: genomics-based insights into the diversity and role of skin microbes. Trends Mol Med 2011; 17: 320–328.
- Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, et al. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014; 2: 6.
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014; 30: 2114–2120.
- Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011; 27: 2957–2963.
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010; 26: 2460–2461.
- MetaStats 2.0 package”: White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol 2009; 5: e1000352.
- Chang HW, Yan D, Singh R, Liu J, Lu X, Ucmak D, et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 2018; 6: 154.
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2013; 486: 207–214.
- Hamady M, Lozupone C, Knight R. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 2010; 4: 17–27.
- Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 2013; 14: 676–684.
- Kosiewicz MM, Zirnheld AL, Alard P. Tuning of skin immunity by skin commensal bacteria. Immunotherapy 2013; 5: 23–25.
- Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124: 1174–1182.
- Rosenbaum JT, Silverman GJ. The microbiome and systemic lupus erythematosus. N Engl J Med 2018; 378: 2236–2237.
- Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR, et al. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med 2012; 186: 536–545.
- Oh PL, Martinez I, Sun Y, Walter J, Peterson DA, Mercer DF. Characterization of the ileal microbiota in rejecting and nonrejecting recipients of small bowel transplants. Am J Transplant 2012; 12: 753–762.
- Han L, Jin H, Zhou L, Zhang X, Fan Z, Dai M, et al. Intestinal microbiota at engraftment influence acute graft-versus-host disease via the Treg/Th17 balance in allo-HSCT recipients. Front Immunol 2018; 9: 669.
- Li K, Mu Z, Wen G, Zhao Y, Cong X, Zhang J. Increased Treg cells and eosinophils characterize atopic dermatitis-like graft-versus-host disease compared to lichen planus-like graft-versus host disease. J Am Acad Dermatol 2020; 83: 824–831.
- Reinhardt K, Foell D, Vogl T, Mezger M, Wittkowski H, Fend F, et al. Monocyte-induced development of Th17 cells and the release of S100 proteins are involved in the pathogenesis of graft-versus-host disease. J Immunol 2014; 193: 3355–3365.
- Varelias A, Ormerod KL, Bunting MD, Koyama M, Gartlan KH, Kuns RD, et al. Acute graft-versus-host disease is regulated by an IL-17-sensitive microbiome. Blood 2017; 129: 2172–2185.
- Quan C, Chen XY, Li X, Xue F, Chen LH, Liu N, et al. Psoriatic lesions are characterized by higher bacterial load and imbalance between Cutibacterium and Corynebacterium. J Am Acad Dermatol 2020; 82: 955–961.
- Ahmed EB, Wang T, Daniels M, Alegre ML, Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant 2011; 11: 936–946.
- Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant 2010; 10: 1524–1533.
- Fyhrquist N, Ruokolainen L, Suomalainen A, Lehtimaki S, Veckman V, Vendelin J, et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. J Allergy Clin Immunol 2014; 134: 1301–1309 e1311.