Prurigo nodularis (PN) features hyperkeratotic and intensely pruritic multiple nodules that mainly appear on the extensor surfaces of the limbs and the trunk. Itch in PN impairs patients’ quality of life and is the most burdensome aspect of this disease’s symptoms instead of the visibility of skin lesion (1). However, treating itch in PN is challenging, because its etiology is largely unknown (2).
Several itch mediators that are Th2-mediated are reported to be responsible for PN-associated itch, including interleukin (IL)-31 and its receptor subunits (IL-31 receptor a (IL-31RA) and oncostatin M receptor β (OSMRβ)) (3), and thymic stromal lymphopoietin (TSLP) and TSLP receptor signaling (4). Recently, an extracellular matrix protein named periostin that promotes a Th2 allergic inflammation has been identified as a novel itch mediator (5). Therefore, we sought in this study to elucidate whether periostin is correlated with itch in PN.
MATERIALS, METHODS, AND RESULTS
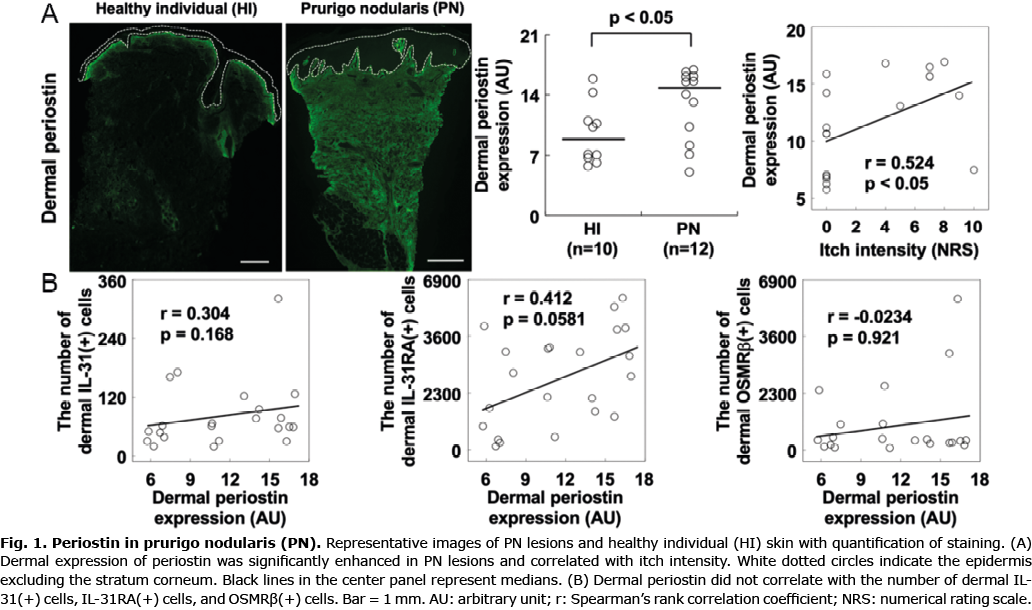
This study was approved by the Institutional Review Board of the University of Miami (20180898). We collected lesional skin samples and clinical data from 12 PN patients (59 ± 15 years (19–77); 4 males and 8 females) and 10 healthy individuals (38 ± 13 years (20–67) ; 5 males and 5 females) at the University of Miami Dermatology outpatient clinic. Their diagnoses were confirmed based on clinical and histological findings. Itch intensity was measured with numerical rating scale (NRS) scores from zero (no itch) to 10 (the worst itch) at the time and location of the biopsy. NRS scores were obtained from 8 PN patients ranging from 4 to 10 (median, 7; average 7.12), and all healthy individuals had no itch (NRS, zero).
Formalin-fixed paraffin-embedded skin samples of 5-µm thickness were deparaffinized and subjected to antigen retrieval with DAKO target retrieval solution (Dako, Glostrup, Denmark) at 60ºC overnight. Samples were next treated with PBS containing 5% normal donkey serum and 0.2% Triton X-100 at room temperature for 2 hours. Then, incubated with primary Abs for periostin (ab14041, Abcam, Cambridge, UK; dilution, 1:200), IL-31 (ab102750, Abcam; dilution, 1:200), IL-31RA (ab113498, Abcam; dilution, 1:200), and OSMR (LS-B11477, LifeSpan BioSciences, Seattle, WA, USA; dilution, 1:200) at 4ºC overnight, followed by reaction with Alexa Fluor 488-conjugated secondary Ab (Molecular Probes, Eugene, OR, USA) and mount with Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Negative control for periostin staining was performed by omitting primary antibody (Fig. S11). For quantification, photomicrographs were captured with DM6000 microscope (Leica Microsystems, Wetzlar, Germany). The deposition of periostin in the entire dermis was measured as fluorescence intensity in arbitrary units (AU) normalized by area and background fluorescence using Image J software (National Institute of Health, Bethesda, MD, USA). The number of immunoreactive cells near the dermo–epidermal junction (in the papillary and subpapillary dermis) was quantified with Image J software (6). To compare differences between two groups, Mann-Whitney’s U test were used. For detecting correlation, we calculated Spearman’s rank correlation coefficient (r) using a statistical software “EZR” (7). Statistical significance was set at p < 0.05.
We found that dermal deposition of periostin was enhanced in PN compared with healthy controls (Mann-Whitney’s U-test, p < 0.05), but immune-reactivity for periostin was not detected at the epidermal layer. Dermal periostin was significantly correlated with itch intensity (Spearman’s correlation, r = 0.524, p < 0.05) (Fig. 1A). In addition, dermal expression of periostin trended to correlate with dermal IL-31RA(+) cells (Spearman’s correlation, r = 0.412, p = 0.0581), but we did not detect any significant correlation of dermal periostin with dermal IL-31(+) cells and OSMRβ(+) cells (Spearman’s correlation, r = 0.304, p = 0.168, and r = –0.0234, p = 0.921, respectively) (Fig. 1B).
DISCUSSION
Various allergic skin diseases with itch show enhanced dermal expression of periostin, including atopic dermatitis (8), scabies (9), stasis dermatitis (10), and bullous pemphigoid (11). In addition to these diseases, we revealed, for the first time, the direct correlation between dermal periostin and itch intensity in PN. In the skin, not only dermal fibroblasts (8) but also epidermal keratinocytes are capable of secreting periostin (5). We detected massive deposition of periostin in the entire dermis, while no immuno-reactivity was confirmed for periostin at the epidermal layer. Accordingly, a major cellular origin of periostin in PN may be dermal fibroblasts.
Periostin involves both direct and indirect pathways to evoke itch. In a direct pathway, periostin directly acts on itch-sensitive nerve fibers via periostin receptor integrin aVβ3 (5) In an indirect pathway, periostin stimulates immune cells (e.g. macrophages and eosinophils) to release IL-31 and other itch mediators (9, 10). It may depend on a disease type which pathway plays a more active role in periostin-associated itch. The current study showed that dermal periostin was not significantly correlated with other major itch mediators including IL-31, IL-31RA, and OSMRβ in the dermis. This finding may suggest that periostin might have a direct effect on itch in PN; further studies examining the role of periostin in itch are required to elucidate this association.
Periostin is also closely involved in allergic inflammation. PN is a Th2 skewed dermatosis with upregulated lesional expression of Th2 cytokines (12). These cytokines induce periostin production from dermal fibroblasts. Furthermore periostin exacerbates secretion of Th2 cytokines causing a vicious cycle (5, 8, 13). Targeting periostin or its receptor integrin aVβ3 may be a good therapeutic option for not only itch but also inflammation itself in PN.
The key limitation of this study was the relatively small number of the samples and the measurement of protein expression exclusively through immunofluorescence staining analysis. However, this study provides a novel insight into the mechanism of PN-associated itch in regard to periostin, which will help guide management of itch in PN.
ACKNOWLEDGEMENTS
We’d like to thank Zamaneh Mikhak, Rohan Gandhi, John Paolini of Kiniksa Pharmaceuticals, Corp. for providing conception guidance and support for this study.
Funding sources: This study was funded by Kiniksa Pharmaceuticals, Ltd.
Conflicts of Interest: GY is Scientific Board Member of Menlo, Trevi, Sienna, Sanofi, Regeneron, Galderma, Pfizer, Novartis, Bayer, Kiniksa, Eli Lilly, and Ortho. Research support by Pfizer, Sun Pharma, Leo, Menlo, and Kiniksa.
REFERENCES
- Pereira MP, Hoffmann V, Weisshaar E, Wallengren J, Halvorsen JA, Garcovich S, et al. Chronic nodular prurigo: clinical profile and burden. A European cross-sectional study. J Eur Acad Dermatol Venereol 2020; 34: 2373–2383.
- Yosipovitch G. Prurigo nodularis: New treatments on the horizon. J Am Acad Dermatol 2020; 82: 1035–1036.
- Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006; 117: 411–417.
- Zhong W, Wu X, Zhang W, Zhang J, Chen X, Chen S, et al. Aberrant expression of histamine-independent pruritogenic mediators in keratinocytes may be involved in the pathogenesis of prurigo nodularis. Acta Derm Venereol 2019; 99: 579–586.
- Mishra SK, Wheeler JJ, Pitake S, Ding H, Jiang C, Fukuyama T, et al. Periostin activation of integrin receptors on sensory neurons induces allergic itch. Cell Reports 2020; 31: 107472.
- Sanders KM, Nattkemper LA, Rosen JD, Andersen HH, Hsiang J, Romanelli P, et al. Non-histaminergic itch mediators elevated in the skin of a porcine model of scabies and of human scabies patients. J Invest Dermatol 2019; 139: 971–973.
- Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl 2013; 48: 452–458.
- Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 2012; 122: 2590–2600.
- Hashimoto T, Satoh T, Yokozeki H. Pruritus in ordinary scabies: IL-31 from macrophages induced by overexpression of thymic stromal lymphopoietin and periostin. Allergy 2019; 74: 1727–1737.
- Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Mechanisms of itch in stasis dermatitis: Significant role of IL-31 from macrophages. J Invest Dermatol 2020; 140: 850–859.
- Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Pathophysiological mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol 2020; 83: 53–62.
- Park K, Mori T, Nakamura M, Tokura Y. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol 2011; 21: 135–136.
- Shiraishi H, Masuoka M, Ohta S, Suzuki S, Arima K, Taniguchi K, et al. Periostin contributes to the pathogenesis of atopic dermatitis by inducing TSLP production from keratinocytes. Allergy Int 2012; 61: 563–572.