ORIGINAL REPORT
Prevalence and Characteristics of Hidradenitis Suppurativa in the Northern Finland Birth Cohort 1986 Study: A Cross-sectional Study of 2,775 Subjects
Suvi-PÄIVIKKI SINIKUMPU1,2, Jari JOKELAINEN3 and Laura HUILAJA1,2
1Department of Dermatology, University Hospital of Oulu, 2Medical Research Center, Research Unit of Clinical Medicine, University of Oulu and 3Northern Finland Birth Cohorts, Arctic Biobank, Infrastructure for Population Studies, Faculty of Medicine, University of Oulu, Oulu, Finland
A negative stigmatization related to hidradenitis suppurativa may prevent patients from seeking care. Thus, a large proportion of patients with hidradenitis suppurativa may be missing from studies based on hospital data. The aim of this study was to examine the prevalence and characteristics of hidradenitis suppurativa among 2,775 subjects in the Northern Finland Birth Cohort 1986 Study (NFBC1986), who were in their mid-thirties. The prevalence of hidradenitis suppurativa was 4.0% (n = 110/2,775), being higher in females (4.8%) than in males (2.5%) (p < 0.01). Of those defined as having hidradenitis suppurativa in this study, only 4 cases (n = 4/110, 3.6%) were found to have a hidradenitis suppurativa diagnosis either in the hospital (Care Register for Health Care) or in the primary healthcare data. In an adjusted model in logistic regression analyses, hidradenitis suppurativa was significantly associated with obesity (body mass index 30–55 kg/m2) (odds ratio 3.81, 95% confidence interval 2.80–5.22), female sex (1.99, 1.53–2.61) and smoking (1.56, 1.21–2.00). In addition, there was an association between hidradenitis suppurativa and self-reported poor overall health status. Hidradenitis suppurativa seems to be common at the population level and only a minority of these patients seek care for the condition.
Key words: hidradenitis suppurativa; prevalence; characteristics; risk factors; overall health; general population; cohort study.
SIGNIFICANCE
Hidradenitis suppurativa is a chronic inflammatory disease of hair follicles, characterized by painful oozing lesions. Due to negative stigmatization of the disease, patients with hidradenitis suppurativa do not easily seek help from physicians. This study examined the prevalence and characteristics of hidradenitis suppurativa in the Northern Finland Birth Cohort 1986 Study (n = 2,775). The study found that hidradenitis suppurativa affected 4.0% of the population. Only 4 cases of the study population were found to have diagnosis of hidradenitis suppurativa, either in hospital or in primary healthcare data. Hidradenitis suppurativa was associated with severe obesity, female sex, smoking and poor overall health status.
Citation: Acta Derm Venereol 2024; 104: adv14732. DOI https://doi.org/10.2340/actadv.v104.14732.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Jun 15, 2023; Accepted: Nov 17, 2023; Published: Jan 10, 2024
Corr: Suvi-Päivikki Sinikumpu, Department of Dermatology, Oulu University Hospital, P.B. 20, FIN-90029 Oulu, Finland. E-mail: suvi-paivikki.sinikumpu@oulu.fi
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Hidradenitis suppurativa (HS) is a chronic, recurrent inflammatory disease of hair follicles, characterized by painful, subcutaneous lesions, most commonly located in the inguinal, axillary and anogenital regions (1). HS is one of the most distressing diseases in dermatology, with known impact on quality of life (2). The annual incidence of HS has not been widely studied, and has been reported to be 3.0-6.0/100,000 persons/year in Finland and Minnesota, US. (3, 4). Prevalence of HS varies between 0.1 and 4% (3, 4). HS affects women more frequently than men (female:male ratio 2:1–5:1) and the incidence is highest among young women (the highest reported prevalence is among those aged 20–39 years) (4–6). HS is associated with many comorbidities, such as cardiovascular diseases, inflammatory bowel disease, and depression (3, 7).
Although knowledge of HS and its associative comorbidities has increased in recent years there is little epidemiological data about the disease. In particular, there is a lack of epidemiological studies performed in the general population (8). Registry studies have large datasets as their strength; however, cases with mild symptoms may be missing. In addition, as the symptoms of HS are often embarrassing, patients may delay seeking help from a physician (9). Thus, the precise prevalence of HS remains unknown. The aim of this cross-sectional study was to examine the prevalence and characteristics of HS at the population level among 2,775 cases belonging to the Northern Finland Birth Cohort 1986 Study (NFBC1986) in their mid-thirties.
MATERIALS AND METHODS
The NFBC1986 Study is a population-based, longitudinal cohort study and research programme. Initially, the NFBC1986 included all 9,749 (9,432 live born) children in the 2 northernmost provinces of Finland whose expected days of birth fell between 1 July 1985, and 30 June 1986 (covering 99% of the children in that area) (10). The cohort subjects have been evaluated regularly since birth by means of health questionnaires and clinical examinations. In 2019 to 2020, at the age of 33–35 years, cohort members were invited to participate in a large follow-up study. In connection with that study the cohort members answered diverse health questionnaires regarding health and health-related factors.
Assessment of hidradenitis suppurativa
The presence of HS was based on the questionnaires. The study cases were asked: (i) “Have you had an outbreak of boils during the last 6 months in the following areas: axilla, groin, genitals, under the breast or abdomen, buttock or gluteal cleft?” and (ii) “How many boils have you had?”; the number of boils: (a) at least 2, (b) 3–5, (c) 6 or more. HS was defined if there was an affirmative answer to the first question combined with at least 2 boils (Appendix S1). These criteria are modified from validated criteria by Vinding et al. (11).
Assessment of other parameters
In the questionnaires, the study cases self-reported their weight, height, living habits (including smoking status, alcohol use, and physical activity) and their opinion about their current health status. Body mass index (BMI) was calculated as the ratio of weight to height squared (kg/m2). Study participants were classified into 4 groups according to BMI and World Health Organization (WHO) criteria: underweight < 18.5 kg/m2; normal weight 18.5–25 kg/m2; overweight 25–30 kg/m2; and obese 30–55 kg/m2. To determine the use of alcohol, the Alcohol Use Disorders Identification Test was used (12). Smoking status was classified as (a) no smoking, (b) former smokers > 6 months (quit smoking less than 6 months ago), (c) former smokers < 6 months (quit smoking more than 6 months ago), and (d) current smoking. In addition, the history of other diseases (both somatic and psychiatric diseases) was asked as follows: “Do you currently have or have you had a below-mentioned doctor-diagnosed disease?” (Appendix S2). By the reported frequency of participation in physical and recreational activities during leisure time, the study cases were classified into 4 groups: Inactive (those who preferred to stay indoors reading or watching television and did not like sports much); Lightly active (those who exercised at least 4 h per week, e.g. by walking, cycling, fishing); Active (those who liked fitness training and had, e.g. running, swimming or skiing as a regular sport activity for at least 2 h per week); Very active (those who exercised several hours per week by running, orienteering or playing ball games. Socioeconomic status (SES) was determined by education: ((a) basic education or high school, (b) vocational education, (c) lower academic education, and (d) upper academic education), and by livelihood status: ((a) regular income, (b) social benefits such as unemployment benefit, (c) student allowance/sickness benefit, (d) no regular income).
Finnish Care Register of Health Care (CRHC)
Patient records (history of HS diagnosed in secondary or public primary care) were obtained from the Finnish Institute of Health and Welfare’s statutory Care Register of Health Care (CRHC) and the AvoHILMO (Care Register for Primary Health Care), and diagnosis of HS was searched using the International Classification of Diseases (ICD) codes 7058C and L73.2. The CRHC contains inpatient data from all state-administered Finnish hospitals and from the largest private hospitals from 1987 onwards and outpatient visits from 1998 onwards. Outpatient visits in primary healthcare are included since 2011. Each record contains the identification numbers of the patient and hospital, primary and subsidiary diagnoses, and duration of hospital stay.
Statistical analyses
The characteristics of the study population are presented as proportions, means and medians. The two-sample t-test and Person’s χ2 test were used to compare the distribution between HS groups. A logistic regression analysis was used to examine associations between HS and risk factors with crude (OR) and adjusted odds ratios (aOR) and 95% confidence intervals (95% CIs). The following potential confounding factors were adjusted for: sex, comorbidities, smoking, and overall health status. An additional adjustment was performed for BMI. The inverse probability weighting, utilizing propensity score methods were used, to generate sampling weights for survey respondents that accurately represent the eligible cohort, in multivariate logistics regression. Propensity score for participation was conducted using sex and education, socioeconomic and employment status as auxiliary variable. These methods were used to correct the participation bias, since there was over-representation of highly educated women in the cohort (personal communication with Nordström et al.). OR was used as a relative risk. The data were analysed using R software package version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was considered statistically significant.
RESULTS
In connection with a 33- to 35-year follow-up study, health questionnaires were sent to n = 8,896 study cases. Of these, n = 2,831 responded to the questionnaires (31.8%); 62.9% of respondents were female. There was some missing data (n = 56) and, thus, the final study population included n = 2,775 cases.
The prevalence of HS was 4.0% (n = 110/2,775), being higher in females (4.8%) than in males (2.5%) (p < 0.01) (Table I). The majority of the patients (n = 90/110, 81.8%) had had 3 or more boils during the previous 6 months. Of those defined as having HS in this study, 4 cases (n = 4/110, 3.6%) were found to have a diagnosis of HS, either in hospital (CRHC) or in primary healthcare data.
| HS (N = 110) | No HS (N = 2,665) | Total (N = 2,775) | p-valueb | |
| Sex, n (%) | 0.002 | |||
| Male | 25 (22.7) | 990 (37.1) | 1,015 (36.6) | |
| Female | 85 (77.3) | 1,675 (62.9) | 1,760 (63.4) | |
| How satisfied are you with your current situation in life in general? n (%) | 0.002 | |||
| Very satisfied | 9 (8.3) | 499 (18.9) | 508 (18.4) | |
| Somewhat satisfied | 63 (57.8) | 1,608 (60.8) | 1,671 (60.7) | |
| Somewhat dissatisfied | 33 (30.3) | 495 (18.7) | 528 (19.2) | |
| Very dissatisfied | 4 (3.7) | 41 (1.5) | 45 (1.6) | |
| Cannot say | 0 (0.0%) | 3 (0.1%) | 3 (0.1) | |
| BMI, kg/m2 | < 0.001 | |||
| Mean (SD) | 29.5 (6.9) | 25.9 (4.8) | 26.1 (5.0) | |
| Median (Q1, Q3) | 27.9 (24.0, 34.6) | 25.0 (22.8, 28.0) | 25.1 (22.8, 28.2) | |
| Min–Max | 19.5–59.1 | 14.9–56.8 | 14.9–59.1 | |
| BMI according to WHO criteria, n (%) | < 0.001 | |||
| Underweight | 0 (0.0) | 32 (1.2) | 32 (1.2) | |
| Normal weight | 33 (30.3) | 1,265 (47.8) | 1,298 (47.1) | |
| Pre-obesity | 31 (28.4) | 924 (34.9) | 955 (34.6) | |
| Obesity | 45 (41.3) | 427 (16.1) | 472 (17.1) | |
| Alcohol use according to AUDIT, n (%) | 0.360 | |||
| Low-risk consumption (score 0–7) | 75 (75.0) | 1,787 (78.7) | 1,862 (78.5) | |
| Hazardous or harmful alcohol consumption (score 8–13) | 18 (18.0) | 391 (17.2) | 409 (17.2) | |
| Likelihood of alcohol dependence (scores 14–) | 7 (7.0) | 94 (4.1) | 101 (4.3) | |
| Smokingc, n (%) | 0.006 | |||
| No | 27 (24.5) | 949 (35.8) | 976 (35.3) | |
| Former smoker > 6kk | 37 (33.6) | 936 (35.3) | 973 (35.2) | |
| Former smoker < 6kk | 11 (10.0) | 124 (4.7) | 135 (4.9) | |
| Current smoker | 35 (31.8) | 644 (24.3) | 679 (24.6) | |
| Livelihood status, n (%) | 0.967 | |||
| Regular income | 85 (77.3) | 2,111 (79.2) | 2,196 (79.1) | |
| Social benefits | 10 (9.1) | 218 (8.2) | 228 (8.2) | |
| Study grants/nursing leave benefits | 13 (11.8) | 287 (10.8) | 300 (10.8) | |
| No regular income | 2 (1.8) | 49 (1.8) | 51 (1.8) | |
| Education, n (%) | 0.247 | |||
| Basic education or high school | 15 (13.6) | 229 (8.6) | 244 (8.8) | |
| Vocational education | 34 (30.9) | 800 (30.2) | 834 (30.2) | |
| Lower academic education | 38 (34.5) | 928 (35.0) | 966 (35.0) | |
| Upper academic education | 23 (20.9) | 694 (26.2) | 717 (26.0) | |
| Civilization, n (%) | 0.474 | |||
| Divorced/judicial separation/single | 43 (39.1) | 948 (35.7) | 991 (35.9) | |
| Relationship | 67 (60.9) | 1,704 (64.3) | 1,771 (64.1) | |
| Physical activity, n (%) | 0.069 | |||
| Inactive | 32 (29.6) | 551 (20.9) | 583 (21.3) | |
| Lightly active | 45 (41.7) | 1,039 (39.4) | 1,084 (39.5) | |
| Active | 29 (26.9) | 958 (36.4) | 987 (36.0) | |
| Very active | 2 (1.9) | 86 (3.3) | 88 (3.2) | |
| aThere may be some missing data while all study cases did not answer to all questions. bTwo sample t-test otherwise χ2 test. cSmoking status: former smokers > 6 months (quit smoking less than 6 months ago), former smokers < 6 months (quit smoking more than 6 months ago). | ||||
| HS: hidradenitis suppurativa: BMI: body mass index; SD: standard deviation; WHO: World Health Organization; AUDIT: Alcohol Use Disorders Identification Test. | ||||
The most common localization of boils was genitals (53.6%), seen more commonly in females (61.2%) than males (28.0%) (p < 0.01). In addition to the genital area, groins (57.6%) were a typical site of boils in females. In males, the buttocks were the most usual site (48.0%) of HS, followed by axilla (36.0%) and groins (36.0%).
There was more smoking (31.8%) and obesity (41.3%) among those with HS than among those without (24.3% and 16.1%, respectively) (p < 0.001 and p < 0.05, respectively). Study cases with HS also had more alcohol use and less physical activity compared with their controls, but the difference was not statistically significant. There was no statistical difference in SES between cases and controls (Table I).
The cases with HS reported their overall health to be significantly worse than the controls (p < 0.01) and they more commonly reported both somatic and psychiatric comorbidities than did controls (Table II and Fig. 1). In logistic regression analyses (adjusted with comorbidities, BMI, sex, current health status (patient’s own opinion) and smoking), subjects with obesity (BMI 30–55 kg/m2) had nearly 4-fold risk (OR 3.81, 95% CI 2.80–5.22) of having HS compared with controls. Correspondingly, female sex (OR 1.99, 95% CI 1.53–2.61) and smoking (OR 1.56, 95% CI 1.21–2.00) increased the risk of HS nearly 2-fold compared with males and non-smokers. There was also a significant association between poor overall health status and HS (OR 2.47, 95% CI 1.21–2.00) (Table III).
| Comorbidities* | HS (N = 110) n (%) | No HS (N = 2,665) n (%) | p-value |
| Cardiovascular diseases | 0.933 | ||
| No | 99 (90.0) | 2,391 (89.8) | |
| Yes | 11 (10.0) | 273 (10.2) | |
| Diabetes | 0.008 | ||
| No | 105 (95.5) | 2,627 (98.6) | |
| Yes | 5 (4.5) | 37 (1.4) | |
| Thyroid diseases | 0.752 | ||
| No | 104 (94.5) | 2,499 (93.8) | |
| Yes | 6 (5.5) | 165 (6.2) | |
| Gastrointestinal diseases | 0.083 | ||
| No | 86 (78.2) | 2,247 (84.3) | |
| True | 24 (21.8) | 417 (15.7) | |
| Neurological diseases | < 0.001 | ||
| No | 68 (61.8) | 2,039 (76.5) | |
| Yes | 42 (38.2) | 625 (23.5) | |
| Malignancies | 0.055 | ||
| No | 103 (93.6) | 2,582 (96.9) | |
| Yes | 7 (6.4) | 82 (3.1) | |
| Psychiatric diseases | 0.019 | ||
| No | 75 (68.2) | 2,070 (77.7) | |
| Yes | 35 (31.8) | 594 (22.3) | |
| Apnoea | 0.027 | ||
| No | 105 (95.5) | 2,619 (98.3) | |
| Yes | 5 (4.5) | 45 (1.7) | |
| Asthma | 0.071 | ||
| No | 90 (81.8) | 2,335 (87.7) | |
| Yes | 20 (18.2) | 329 (12.3) | |
| Chronic obstructive pulmonary disease | 0.463 | ||
| No | 105 (95.5) | 2,577 (96.7) | |
| Yes | 5 (4.5) | 87 (3.3) | |
| Musculoskeletal disorders and rheumatic diseases | 0.375 | ||
| No | 77 (70.0) | 1,966 (73.8) | |
| Yes | 33 (30.0) | 698 (26.2) | |
| *The detailed list of comorbidities is shown in Appendix S1. | |||
| Hidradenitis suppurativa | |||
| Crude odds ratio | Adjusted model 1a | Adjusted model 2b | |
| Comorbidities | |||
| Somatic or psychiatric comorbid | 1.30 (0.84, 2.05) | 1.07 (0.80, 1.43) | 1.07 (0.80, 1.43) |
| p = 0.252 | p = 0.65 | p = 0.67 | |
| Somatic and psychiatric comorbid | 2.14 (1.24, 3.64) | 1.24 (0.86, 1.79) | 1.12 (0.77, 1.62) |
| p = 0.006 | p = 0.25 | p = 0.54 | |
| Sex | |||
| Female | 2.01 (1.30, 3.22) | 1.92 (1.48, 2.50) | 1.99 (1.53, 2.61) |
| p = 0.003 | p = 0.00001 | p = 0.0001 | |
| How satisfied are you with your current situation in life in general? | |||
| Satisfied | 2.17 (1.13, 4.71) | 1.99 (1.26, 3.31) | 1.64 (1.03, 2.73) |
| p = 0.032 | p = 0.01 | p = 0.05 | |
| Somewhat satisfied | 3.70 (1.83, 8.29) | 3.43 (2.11, 5.84) | 2.12 (1.29, 3.66) |
| p = 0.001 | p = 0.0001 | p = 0.005 | |
| Somewhat or very dissatisfied | 5.04 (1.32, 16.17) | 3.76 (1.73, 7.97) | 2.47 (1.12, 5.29) |
| p = 0.010 | p = 0.001 | p = 0.03 | |
| Smoking status | |||
| Current smoker or Former smoker < 6 months | 1.74 (1.17, 2.55) | 1.54 (1.20, 1.97) | 1.56 (1.21, 2.00) |
| p = 0.006 | p = 0.001 | p = 0.001 | |
| Body mass indexc | |||
| 25–30 kg/m2 | 1.32 (0.80, 2.17) | 1.38 (1.00, 1.92) | |
| p = 0.276 | p = 0.06 | ||
| 30–55 kg/m2 | 4.14 (2.62, 6.62) | 3.81 (2.80, 5.22) | |
| p < 0.001 | p < 0.001 | ||
| Logistic regression analyses: aAdjusted by comorbidities, sex, overall health status, smoking. bAn additional adjustment for body mass index. cAccording to the World Health Organization (WHO) classification. | |||
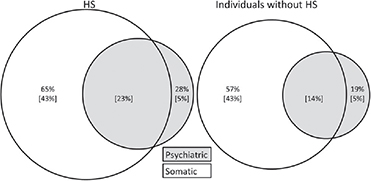
Fig. 1. Prevalence of psychiatric and somatic morbidity in patients with hidradenitis suppurativa (HS) and in those without HS. Values in brackets are the pure or comorbid prevalence of psychiatric or somatic disorders.
DISCUSSION
This study was conducted to chart the prevalence rate of HS in a unique NFBC1986 Study. The prevalence of self-reported HS was 4.0%, which is higher than that reported in larger studies from the USA (0.1%, n = 48 million) (5), France (~1%, n = 6,887) (8) or Denmark (1.8%, n = 27,275) (13). However, the prevalence level in 2 small Danish studies by Jemec and co-workers correspond to ours (~4%) (14, 15). The variation in the prevalence between the current study and previous studies is probably due to the differences in study methodology and study groups. For example, the general US population study included only cases who had sought care in health systems or had been diagnosed with HS (5). The large Danish study, in turn, consisted of blood donors and, thus, the study sample is probably not directly comparable with the general population (13). There were also some heterogeneities in mean age between the studies (8), with ours including only adults in their mid-thirties. Since HS is reported to affect young adults especially (with a female predominance), resembling our study population, the relatively high prevalence in the current study is reasonable. Surprisingly, only very few (n = 4/110) of the subjects with HS in the current study were found to have a healthcare contact regarding HS or diagnosed HS, strengthening the hypothesis that stigmatization related to HS may prevent patients from seeking care. However, since the healthcare data in the current study is registry-based, we cannot exclude the possibility of misdiagnosed HS (i.e. regular boils/abscesses).
In this study, there was a female preponderance in HS (2-fold risk), corresponding to other studies reporting the female-to-male ratio to be as high as 5:1 (6). The exact mechanisms behind the sex difference in prevalence of HS is unknown, but sex hormones are thought to be involved in the pathophysiology; e.g. pregnancy and premenstrual flares are known to exacerbate the symptoms of HS, whereas onset of HS is rare after menopause (16).
The association between obesity and HS is well known from previous studies and the current study finding is consistent with others (4, 13, 17). The mean BMI among HS cases in the current study was 29.5 kg/m2 (overweight) and nearly half (41.3%) of the cases were obese (females significantly more often than males) (4, 13, 17). In a Danish study, the mean BMI among subjects with HS was 27.0 kg/m2 (13), and in a US study, 32.6 kg/m2 (4). Some studies have found over 75% of patients with HS to be obese (17). Obesity can activate HS in many ways; it contributes to an abnormal hormonal metabolic status, causes low-grade inflammation, and, via increased skin-to-skin contact, leads to increased friction, maceration and occlusion, which may result in follicular hyperkeratosis and plugging (17). In addition to obesity, smoking is an important environmental risk factor of HS (17). In the current study, the subjects with HS reported significantly more smoking than controls. Like obesity, smoking influences the structure of the skin (e.g. stimulates follicular hyperplasia and occlusion, modifies sweat gland activity) and produces inflammatory reactions (17, 18).
The subjects with HS in this study considered their overall health to be worse than the controls. The burden of HS is known to be high and parallel to many chronic diseases, such as rheumatoid diseases (21). Correspondingly in the other Finnish study (2) patients with HS (n = 92, patients in tertiary care, evaluated by the generic 15 D questionnaire) had significantly diminished health-related quality of life compared with the general population. It is notable that, in that study, the findings were independent of HS severity (2). HS may be a very embarrassing disease with serious consequences, and patients with HS, especially females, consider the burden of HS to be very high (22), and have also been reported to have an increased risk of suicide regardless of whether they have a previous psychiatric diagnosis (23).
Association of HS with multiple somatic and psychiatric diseases has been shown in several studies (3, 7). In line with these, the current study found higher prevalences of many diseases in our subjects with HS compared with controls. Nevertheless, the association between comorbidities and HS may be modified by other factors, such as sex, smoking and BMI, as seen in our analyses. In particular, BMI was a strong risk factor for HS, which was the reason we performed an additional adjustment for BMI. However, due to the rather small group size, more studies are needed to evaluate specific diseases more accurately.
The major strength of this study is that it is based on a large (nearly 3,000 subjects) birth cohort population. Studies such as this one offer important information about the more realistic prevalence of HS, since they are not focused on selected patient groups: as seen in the current study, only a minority of the subjects with HS were found from the hospital/primary care register data. The participation rate in the study was satisfactory and comparable with the participation rates in other cross-sectional European health examination surveys (24, 25). In addition, due to the study design, we were able to evaluate comprehensively the basic characteristics and comorbidities of the patients with HS. As a limitation, even though the definition of HS in this study was based on the internationally accepted criteria (11), the possibility of misdiagnosis remains (due to self-reporting data) (26). However, self-reported diagnoses are, in turn, also stated to produce the highest estimates of HS prevalence (1). Nevertheless, since Koptyev et al. (26) have questioned the reliability of self-reporting in HS definition, we decided to slightly modify the criteria presented by Vinding and co-workers (11). Furthermore, we studied a narrow age cohort of Caucasian subjects, which may limit the generalization of the current results outside the birth cohort or to other age groups or ethnicities. Finally, not all invited cohort members participated in the study (there was an over-representation of highly educated women (personal communication with Nordström et al.), and, the rate of non-response must be regarded as sample bias. Furthermore, this may have led to some bias, both in the prevalence of HS and in the prevalence of comorbidities studied. However, the inverse probability weighting methods were used to correct this bias.
In conclusion, this study demonstrated a high prevalence of HS in this cohort population among subjects in their mid-thirties. HS was more common in females than in males and was associated with obesity and smoking. Subjects with HS were affected with a high burden of comorbidities and they considered their own health to be poor. Thus, the risk factors of HS in this unselected population seem to correspond to those in hospital register studies (7). It is noteworthy that only a few of the current study cases were registered in healthcare data because of HS, and thus, it is important to remember that HS cases are less likely to seek help for their disease.
ACKNOWLEDGEMENTS
We thank all cohort members and researchers who participated in the 33- to 35-year study. We also wish to acknowledge the work of the NFBC project center.
Data referral: http://urn.fi/urn:nbn:fi:att:f5c10eef-3d25-4bd0-beb8-f2d59df95b8e
A data availability statement: NFBC data is available from the University of Oulu, Infrastructure for Population Studies. Permission to use the data can be applied for research purposes via the electronic material request portal. In the use of data, we follow the EU general data protection regulation (679/2016) and the Finnish Data Protection Act. The use of personal data is based on cohort participants’ written informed consent at their latest follow-up study, which may cause limitations to its use. More information is available by contacting the NFBC project center (NFBC project center (at)oulu.fi) and visiting the cohort website.
The ethics committee of the Northern Ostrobothnia Hospital District approved the present study (§108/2017), which was performed according to the principles of the 1983 Declaration of Helsinki. The subjects took part on a voluntary basis and gave their informed consent. The data was handled on a group level and was pseudonymized for analyses.
REFERENCES
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol 2015; 73: 4–7.
- Hirvonen MJ, Pasternack R, Lipitsä T, Vihervaara A, Harvima R, Ranta M, et al. Patients with hidradenitis suppurativa suffer from low health-related quality of life as measured by the generic 15D instrument. Skin Appendage Disord 2022; 8: 221–227.
- Huilaja L, Tiri H, Jokelainen J, Timonen M, Tasanen K. Patients with hidradenitis suppurativa have a high psychiatric disease burden: a Finnish Nationwide Registry Study. J Invest Dermatol 2018; 138: 46–51.
- Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol 2013; 133: 97–103.
- Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol 2017; 153: 760–764.
- Wiseman MC. Hidradenitis suppurativa: a review. Dermatol Ther 2004; 17: 50–54.
- Garg A, Malviya N, Strunk A, Wright S, Alavi A, Alhusayen R, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol 2022; 86: 1092–1101.
- Revuz JE, Canoui-Poitrine F, Wolkenstein P, Viallette C, Gabison G, Pouget F, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case-control studies. J Am Acad Dermatol 2008; 59: 596–601.
- Garg A, Neuren E, Cha D, Kirby JS, Ingram JR, Jemec GBE, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020; 82: 366–376.
- NFBC 1986 Data Collection. Northern Finland Cohorts. [accessed 2022 May 30] Available from: https://www.oulu.fi/en/university/faculties-and-units/faculty-medicine/northern-finland-birth-cohorts-and-arctic-biobank/research-program-health-and-well-being.
- Vinding GR, Miller IM, Zarchi K, Ibler KS, Ellervik C, Jemec GBE. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol 2014; 170: 884–889.
- Saunders JB, Aasland O, Babor T, de la Fuente J, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction (Abingdon, England) 1993; 88: 791–804.
- Theut Riis P, Pedersen OB, Sigsgaard V, Erikstrup C, Paarup HM, Nielsen KR, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol 2019; 180: 774–781.
- Jemec GB. The symptomatology of hidradenitis suppurativa in women. Br J Dermatol 1988; 119: 345–350.
- Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35: 191–194.
- Riis P, Ring H. Themstrup L, Jemec GB. The role of androgens and estrogens in hidradenitis suppurativa – a systematic review. Acta Dermatovenereol Croat 2016; 24: 239–249.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol 2009; 60: 539–561.
- Mokos ZB, Miše J, Balić A, Marinović B. Understanding the relationship between smoking and hidradenitis suppurativa. Acta Dermatovenerol Croat 2020; 28: 9–13.
- Deckers I, Janse I, van der Zee H, Nijsten T, Boer J, Horváth B, et al. Hidradenitis suppurativa (HS) is associated with low socioeconomic status (SES): a cross-sectional reference study. J Am Acad Dermatol 2016; 75: 755–759.
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health: the challenge of the gradient. American Psychologist 1994; 49: 15–24.
- Kluger N, Ranta M, Serlachius M. The burden of hidradenitis suppurativa in a cohort of patients in southern Finland: a pilot study 2017. Skin Appendage Disord 2017; 3: 20–27.
- Samela T, Dattolo A, Cordella G, Antinone V, Mastroeni S, Fusari R, et al. Similar levels of disease severity correspond to a greater burden of illness in women compared with men with hidradenitis suppurativa. Acta Derm Venereol 2023; 103: adv00856.
- Tiri H, Huilaja L, Jokelainen J, Timonen M, Tasanen K. Women with hidradenitis suppurativa have an elevated risk of suicide. J Invest Dermatol 2018; 138: 2672–2674.
- Nordström T, Auvinen J, Ala-Mursula L, Keinänen-Kiukaanniemi S, Veijola J, Järvelin MR et al. Cohort Profile: 46 years of follow-up of the Northern Finland Birth Cohort 1966 (NFBC1966). Int J Epidemiol 2021; 50: 1–12.
- Mindell JS, Giampaoli S, Goesswald A, Kamtsiuris P, Mann C, Mannisto S, et al. Sample selection, recruitment and participation rates in health examination surveys in Europe – experience from seven national surveys. BMC Med Res Methodol 2015; 15: 74–78.
- Koptyev J, Strunk A, Garg A. Low predictive value of questionnaire-based diagnosis of hidradenitis suppurativa. Br J Dermatol 2022; 187: 1015–1017.