The effect of sex on systemic therapy for psoriasis has not been well studied. The aim of this study was to analyse a large multicentre Spanish cohort of 2,881 patients with psoriasis (58.3% males), followed from January 2008 to November 2018, to determine whether sex influences prescription, effectiveness of therapy, and the risk of adverse events. The results show that women are more likely than men to be prescribed biologics. There were no differences between men and women in effectiveness of therapy, measured in terms of drug survival. Women were more likely to develop adverse events, but the difference in risk was small and does not justify different management. Study limitations include residual confounding and the use of drug survival as a proxy for effectiveness.
Key words: gender; sex; gender bias; sex bias; psoriasis; biological therapy; drug prescription; drug safety.
Accepted Nov 27, 2020; Epub ahead of print Dec 3, 2020
Acta Derm Venereol 2021; 101: adv00354.
doi: 10.2340/00015555-3711
Corr: Carlos Pelayo Hernández-Fernández, Fundación Piel Sana AEDV, Calle Ferraz 100, ES-28008, Madrid, Spain. E-mail: pelaezeleven@gmail.com
SIGNIFICANCE
The effect of sex on systemic therapy for psoriasis has not been well studied. The aim of this study was to determine, in a large group of 2,881 patients followed from January 2008 to November 2018, whether sex influences prescription, effectiveness of therapy, or the risk of adverse events. The results show that women were more likely than men to be prescribed systemic therapy. No differences between men and women were found in the effectiveness of therapy. Women were also more likely to experience adverse events, but the difference in risk is small, and does not justify different management.
INTRODUCTION
Sex differences are recognized in medicine (1), and analysis of inequalities in practice care may be based on these differences (1). Sex is usually treated as a potential confounder, ignoring whether results apply to both males and females, and excluding the analysis of differences according to sex itself. While there is increasing interest in developing studies to completely integrate the analysis of sex (2), within dermatology, sex perspective is still an opportunity to identify disparities (3) in order to improve equality and efficiency of care.
Psoriasis affects 2–3% of the general population. Although men and women are equally affected in terms of prevalence (4), sex differences have been observed concerning prescription, and effectiveness and safety of systemic treatment (5–16).
Some studies have shown that men are more frequently treated with systemic and biological drugs than are women, which has been linked to the greater severity of the disease in men (5, 7). Other publications have demonstrated that women and men experience the social and mental impact of psoriasis differently (8, 9). Sex differences are seen in the subjective disease scores for psoriasis, with women achieving worse scores than men, regardless of treatment (6, 10). Various publications have shown that, irrespective of the severity of the disease, women are more prone than men to depressive symptoms, psychological distress and impairment of quality of life (11).
Concerning the safety of systemic therapy in psoriasis, several studies indicate female sex as a predictor for discontinuation of biologic therapy due to a higher frequency of side-effects (6, 12, 13). Nevertheless, these studies have limitations, since many are based on drug survival analyses, which may not be a good instrument for measuring the safety of treatments (14).
In order to identify possible differences concerning systemic psoriatic therapy used in daily practice care by female and male patients, the aim of this study was to compare prescription, effectiveness and safety between the sexes in the BIOBADADERM cohort.
MATERIALS AND METHODS
A detailed description of BIOBADADERM has been published previously (15, 16). Established in 2008 as the Spanish prospective cohort of patients with moderate-to-severe psoriasis receiving systemic therapy, it is aimed at describing long-term safety. All consecutive patients in each centre treated with modern (other than classical) systemic drugs are invited to enter the cohort, as well as the next patient who receives a classical systemic drug (acitretin, cyclosporine, methotrexate). Sixteen dermatology departments, distributed throughout the country, participated in this study. This analysis included all prospective patients from January 2008 through November 2018, excluding patients in combination therapy, due to the difficulty of attributing the results obtained to a single drug.
Adverse events (AE) were collected using the Medical Dictionary for Regulatory Activities (MedDRA). Patients were contacted at least once a year, although more frequent visits were usual as part of standard care. All AE were included in the database if they were serious or led to a change in therapy or to an unplanned healthcare demand. Serious AE (SAE) were those that were life-threatening, required prolonged hospitalization, caused persistent disability or resulted in death. Drug exposure to systemic therapy was measured from the start of treatment to the date of last administration, or to the censor date in patients who were lost to follow-up. Patients who were lost to follow-up were censored at the last visit to the dermatologist.
BIOBADADERM is monthly monitored online, and once a year data is validated by on-site audits. BIOBADADERM was approved by the Hospital 12 de Octubre Ethics Committee (216/07) and patients gave their written informed consent.
Study groups and outcomes
The main exposure was sex: males and females. In addition, systemic treatment was further divided into classical therapies (acitretin, cyclosporine, methotrexate) and modern therapies, including biologic and small molecules (etanercept, infliximab, adalimumab, ustekinumab, secukinumab, ixekizumab and apremilast). The main outcomes were treatment prescription, effectiveness and safety. Prescription was considered as the difference in the odds of use of classical compared with modern therapies. Due to the lack of other effectiveness measures, the probability of treatment discontinuation, due to ineffectiveness or remission, under a competing risks scenario, was considered a proxy measure for effectiveness. Safety was measured using relative risks and risk differences for AE.
Statistical analysis
Descriptive data were expressed as absolute numbers and percentages for discrete variables, and as medians and interquartile ranges for continuous variables. Results between males and females were compared using the Pearson’s χ2 test or Fisher’s exact test for qualitative variables, and the Wilcoxon–Mann–Whitney test for quantitative variables. For multivariable analysis, individual drugs (not overall categories) were included in the analyses.
Propensity scores
A propensity score (PS) was estimated in order to reduce the selection bias from non-random allocation of treatments in cohort studies. PS was created based on the probability of indication for classical against modern therapies and was obtained by building a logistic regression model, using all variables potentially associated with treatments and outcomes as independent variables (17). PS was incorporated as a confounder in the analysis of all outcomes, except for the analysis of prescription, since the aim of the current study was not to control for the difference in the use of treatments, but to detect it.
Missing values analysis
Most of the variables analysed had no relevant missing data. Some comorbidities had missing data, most ranging between 4% (e.g. hypertension) and 6% (e.g. chronic liver disease). The highest percentage was for alcohol consumption (23%).
Five complete datasets were created by means of chained equations, assuming that missing values were missing at random, using a fully conditional specification model (18). Missing values were imputed using other T individual’s observed variables. Imputed values were examined using iteration to assess convergence and stationarity of each chain. The 5 datasets were analysed using specific regression models for every outcome. Finally, the results of the complete datasets were combined into a single set of estimates using the Rubin rules (19).
Prescription
A nested case-control design with incidence density sampling was used. Prescription was the outcome and sex was the exposure. For this analysis, a multilevel mixed-effects logistic regression model was built to determine the association between modern systemic therapy and sex. The hospital was considered as a random effect, due to within-centre clustering of patients. Firstly, crude results were obtained with univariate regression analysis, using treatment as outcome, sex as exposure, and demographic characteristics (age, body mass index (BMI), smoking and alcohol consumption), clinical characteristics (disease duration, Psoriasis Area and Severity Index (PASI), type of psoriasis and psoriatic arthritis), comorbidities and previous treatments (number of previous systemic classical treatment, previous phototherapy and treatment order) as independent variables. A backward selection multivariate model was then constructed to adjust for confounders, using variables potentially associated with prescription in the univariate model.
Effectiveness
Using the cohort design, survival of first drug was measured as a proxy for effectiveness in a competing risk survival scenario. Competing risks regression models were used to compare every specific subhazard ratio (SHR) for ineffectiveness or remission (similarly interpreted to hazard ratios in Cox regression) and cumulative incidence functions (CIF) of discontinuation. AE, remission and ineffectiveness were considered as main competitors, whereas all other reasons for discontinuation (e.g. lost to follow-up, patient’s decisions) were considered as right censoring (20). Subhazard ratios were estimated firstly in crude models, and then built by means of a backward selection multivariate model, using the same potential confounders as in prescription analysis, plus the PS. CIF were represented showing the probability of specific withdrawal over time.
Safety
Crude and adjusted incidence rate ratios (aIRR) and incidence rate differences (IRD, similarly interpreted as risk differences) were estimated for all AE, SAE, fatal events and each MedDRA system organ class group compared between males and females. For aIRR, a mixed-effects Poisson regression model was used, considering the hospital as a random effect. Age, psoriatic arthritis, specific treatment, cycle order and PS were included in the final model as the main confounders, according to univariate models and previous results (16). Incidence rates of AE per 1,000 patient-years of exposure by period were also described.
All analyses were performed using STATA v.14.2 (Stata Corp. (College Station, TX, USA) 2015. Stata Statistical Software: Release 14). A p-value < 0.05 was considered statistically significant.
RESULTS
A cohort of 2,881 patients with psoriasis treated with systemic therapy was analysed, of which 1,680 (58.3%) were male and 1,201 (41.7%) were female; of the latter, 56% were women of reproductive age (15–49 years). Median PASI at baseline was higher in men (11.2 vs 9, p < 0.0001). Almost all patients had plaque psoriasis, although women had a higher frequency of guttate and palmoplantar pustular psoriasis (p < 0.0001). Twelve percent of the patients had psoriatic arthritis, with no differences according to sex. Other demographic and baseline clinical patient characteristics are shown in Table I.
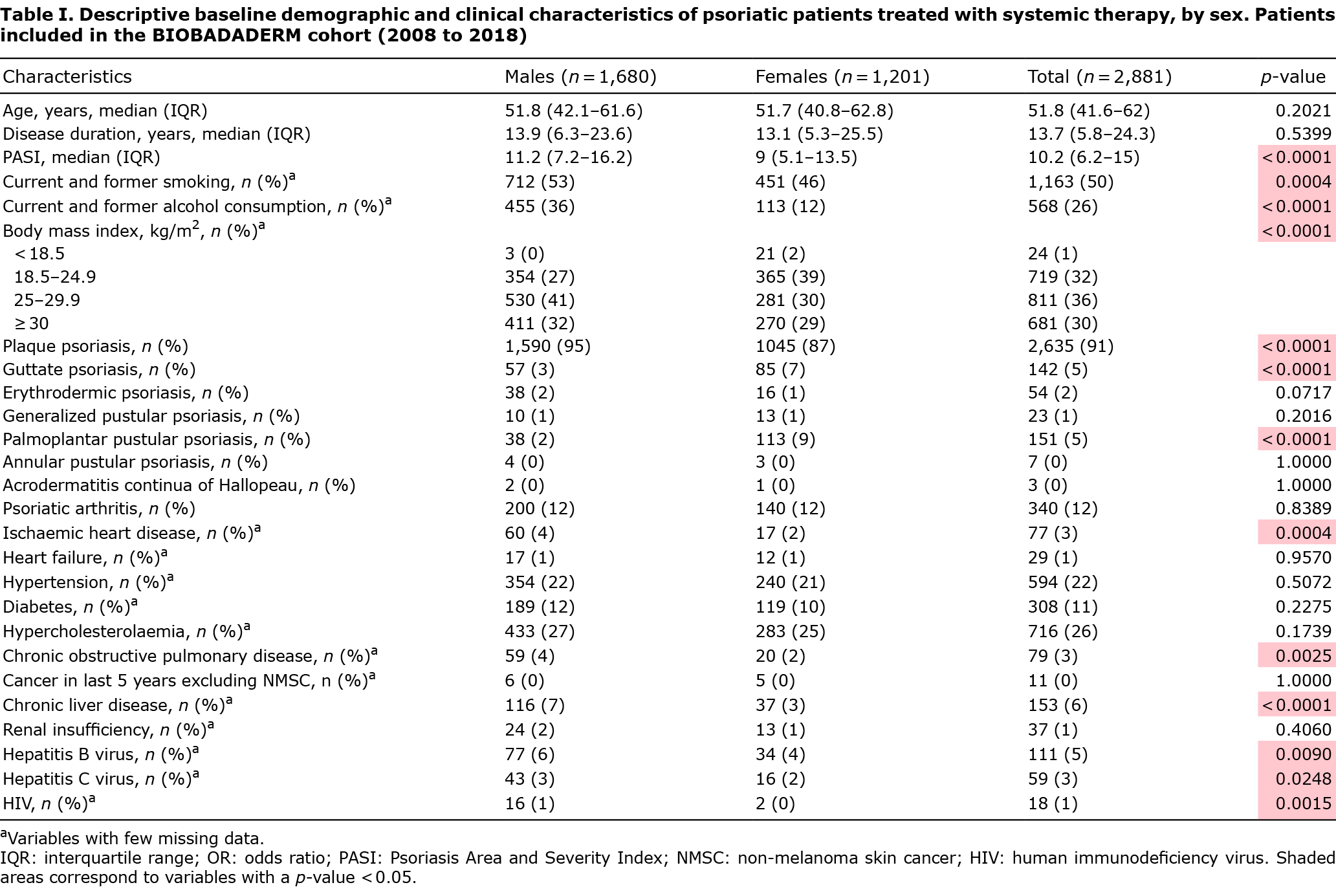
Most patients (54%) had tried at least one classical treatment prior to entry in the cohort, whereas only 11% had tried modern treatment (biologics or apremilast). Thirty-eight percent of patients had tried previous phototherapy. A majority of patients (53%) had received at least 2 drugs. Regarding both classical and modern therapy, methotrexate was the drug most often used as first treatment (26%), followed by etanercept (15%). Regarding all current and past treatments, methotrexate was also the most frequent (23%). One out of every 2 patients received current modern therapy. Additional information is shown in Table SI.
Prescription
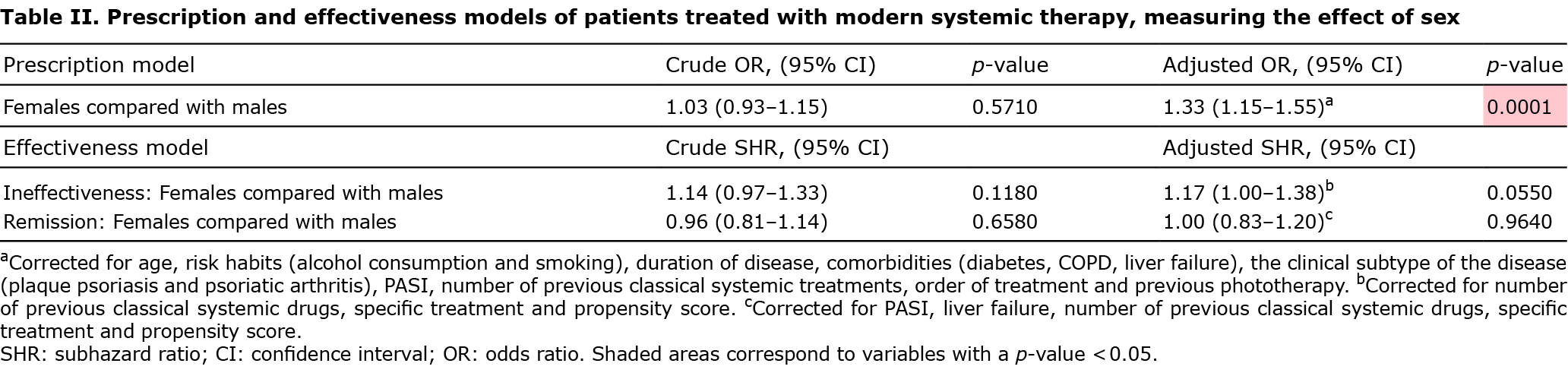
Crude analysis in Table II shows that males were not more likely than females to be prescribed modern systemic therapy (odds ratio (OR) 1.03; 95% confidence interval (95% CI) 0.93–1.15). However, when the adjusted odds of prescription were compared, females had a 33% higher chance than males of being prescribed modern therapy (OR 1.33; 95% CI 1.15–1.55). The results were corrected for those significant possible confounders, including age, risk habits (alcohol consumption and smoking), duration of disease, comorbidities (diabetes, chronic obstructive pulmonary disease (COPD), liver failure), the clinical subtype of the disease (plaque psoriasis and psoriatic arthritis), PASI, number of previous classical systemic treatments, treatment order and previous phototherapy.
Effectiveness
No correlation was found between sex and risk of discontinuing the first treatment due to clinical ineffectiveness, neither crude nor corrected for significant confounders, such as the number of previous classical systemic drugs, specific treatment and propensity score (SHR 1.17; 95% CI 1.00–1.38; p = 0.055). There were also no differences related to suspension due to remission of the disease, neither crude nor corrected for PASI, number of previous classical systemic drugs, liver failure, specific treatment and PS (SHR 1.00; 95% CI 0.83–1.20; p = 0.964) (Table II).
Cumulative incidence curves in competing risks of ineffectiveness and remission over time are shown in Fig. 1. No statistical differences (p > 0.05) were observed between males and females.

Safety
Table III summarizes the differences in the rates of specific AE between males and females. The aIRR of AE was significantly higher in women, including both the overall rate (1.37, 95% CI 1.3–1.44) and the rate of SAE (1.28, 95% CI 1.09–1.51), leading to a risk difference (95% CI) of 232 (198–266) and 16 (5–26) events per 1,000 patient-years, respectively. However, the adjusted incidence rate of fatal AE was slightly lower in women, with a non-statistically significant effect (aIRR 0.55, 95% CI 0.3–1.01).

DISCUSSION
The main findings of this multicentre prospective study with a wide national cohort of psoriatic patients undergoing systemic treatment, are that: (i) women are more likely than men to be prescribed biologics; (ii) effectiveness seems to be similar in both groups; and (iii) AE are more common in women and associate with a different profile to that of men. Although researchers usually keep in mind the role that sex can play as a potential confounder, these findings highlight the fact that analysing results by sex itself is valuable.
Concerning psoriasis severity, men had more severe disease at baseline (median PASI 11.2 vs 9), as reported by other studies. Of note, only 12% of the patients in the current cohort had psoriatic arthritis (PsA), with no differences between men and women. Although wide-ranging prevalence estimates of PsA in patients with psoriasis have been reported in the literature, a recent meta-analysis found a prevalence of 22.7% among European patients (21). This difference could be due to real differences in prevalence between Spain and other countries. It could also be related to an information bias, due to lack of assessment of arthritis symptoms during patients’ follow-up, resulting in under-diagnosis of PsA. Another possible reason is the exclusion of patients in combination therapy, since good control of both cutaneous psoriasis and PsA often requires multidrug therapy. Nevertheless, the prevalence in men and women was exactly the same; hence PsA is unlikely to explain the disparities found in prescription and safety.
Regarding prescription, this study found that women have a 33% higher probability of being treated with a modern drug (mostly biological therapy) than do men, once confounding factors are taken into account, including disease severity, which tends to be greater in males. A possible reason may be that, as shown in other studies, women tend to subjectively perceive a higher severity of the disease (6, 11, 22, 23), which could translate into higher expectations and demand for modern therapy (24). It is also possible that dermatologists tend to avoid using classical systemic drugs in young fertile and pregnant women because of their potential adverse effects on pregnancy. A sensitivity analysis (Table SII) was performed to evaluate the association between age and prescription-effectiveness of modern therapy, dividing the population into 2 groups: 20–40 years and > 40 years of age. The odds ratio (OR) of prescription was similar to that of the whole population, suggesting that age does not influence prescription in women.
Hägg et al. (5, 7) analysed the Swedish national registry of systemic treatments in psoriasis (PsoReg) to describe the time to prescription of a biologic. They found that 63% of patients treated with biologics were male. However, time to biologic prescription was similar in males and females when confounding factors were taken into consideration (age, BMI, presence of arthritis and PASI). More common prescription in males was attributed to men with more severe psoriasis. The current study included more relevant potential confounders, such as risk habits, comorbidities or previous classical systemic treatments, which could explain the different results. It is also possible that sex differences in prescription vary across countries.
Effectiveness was measured using drug survival as a proxy measure, as the current dataset and study design preclude better effectiveness outcomes from being used. Although this method has some drawbacks (14), the current study aimed to minimize them by selecting only survival for specific outcomes in a competing risk scenario, and including the drug in the model. In terms of suspension due to remission or ineffectiveness, no significant differences associated with sex were found. Warren et al. (25) analysed the British Association of Dermatologists Biologic Interventions Register (BADBIR), finding that female sex was associated with reduced odds of achieving ≥ 90% improvement in PASI at 6 and 12 months. However, this was estimated in a multivariable model, in which sex was not being studied as the main exposure, thus it should be interpreted with caution (26). Similarly, Richter et al. (27) identified male sex as a positive predictor of longer adalimumab survival in psoriasis, and Pogácsás et al. (28) observed more long-term survival of TNF-alpha inhibitors and ustekinumab amongst men in a Hungarian cohort. Given the differences in methodologies, further prospective studies should be carried out to measure the impact of sex on the effectiveness of systemic therapies. It would also be useful if randomized clinical trials (2) and systematic reviews (29) provided results by sex, as mentioned previously.
Although the vast majority of cases (91%) were plaque psoriasis, the current study aimed to rule out the effect of the specific type of psoriasis in prescription and effectiveness of treatment. Since guttate psoriasis was the second most common type of psoriasis (5%), we carried out a sensitivity analysis (Table SIII) comparing patients with only plaque and guttate psoriasis vs all patients, finding similar results in both groups.
Finally, a 37% higher rate of overall AE and 232 more AEs per 1,000 person-years was found in females. Adverse events were higher in women in all system groups of disease, except in investigations, metabolism, and nutrition disorders and cardiac disorders. This is striking, since women are treated with biological therapy more often than men, rather than classical drugs, and we would expect to find a greater risk of AE in the latter, in contrast to the newer and more targeted biologics. This could be related to the fact that, as mentioned above, women tend to perceive a higher severity of the disease and demand a more intensive follow-up, which could translate into a higher rate of self-reporting of AE. However, this needs to be interpreted with caution, since serious AE were also more frequent in women than men, suggesting that women are indeed more likely to develop AE.
The BioCAPTURE registry described that women were less satisfied and experienced AE more frequently than did men (6). Di Cesare et al. (30) found that female sex itself was a risk factor for acute infective events in a cohort of patients with stable chronic plaque psoriasis. Similarly, Zweegers et al. (11) observed that female sex was a predictor for discontinuation of adalimumab, etanercept and ustekinumab due to AE. Similarly, in a recent systematic review, Mourad et al. (31) identified female sex as a predictor of biologic withdrawal owing to AE. An accurate description of safety in women was compiled in the current study, as the study cohort was specifically designed to describe and collect AE, and the current results align with those in the literature. Propensity scores were used to avoid prescription bias. Previous studies have shown that using survival as a proxy outcome for safety can be misleading (14).
Study strengths and limitations
The main strength of this study is that it analyses a large multicentre prospective cohort formed by patients treated in daily practice care, with long follow-up times. It also comprises a relatively large number of different hospitals throughout the country, where quality of data is constantly monitored. Although residual confounding cannot be totally ruled out from observational studies, a large number of variables were collected that allowed us to control the effect of potential confounders. With the same purpose, the use of propensity scores, when appropriate, helped us to avoid prescription bias. Its limitations are the usage of drug survival as a proxy for effectiveness and the lack of information regarding specific variables of interest, such as the level of satisfaction with the treatment or the benefits of treatment in terms of quality-adjusted life years. As mentioned previously, the current study excluded patients in combination therapy due to the difficulty of attributing the results to a specific drug, and this may have acted as a limitation.
Conclusion
These findings indicate that there may be a sex distinction in prescription of biological drugs in favour of females. Effectiveness, measured as drug survival, seems to be similar in both sexes, either in terms of suspension due to remission or ineffectiveness. We have found that women have a greater risk of developing serious and global AE. Despite the contrast in prescription and safety, these differences are relatively small and should not prompt a different follow-up and management between males and females. These results emphasize the need to consider sex as a valuable factor in psoriasis systemic therapy decision-making in routine practice care. They also highlight the importance of analysing and presenting results of studies by sex.
ACKNOWLEDGEMENTS
This work was conducted within the BIOBADADERM Study Group. The following members participated in acquisition of data and review of the manuscript: Esteban Daudén, Mar Llamas-Velasco (Hospital Universitario de la Princesa); Gregorio Carretero, Jaime Vilar-Alejo (Hospital Universitario de Gran Canaria Dr. Negrín); Raquel Rivera, Carmen García-Donoso (Hospital Universitario 12 de Octubre); Carlos Ferrándiz, José Manuel Carrascosa, Ferrán Ballescá (Hospital Universitari Germans Trias i Pujol); Pablo de la Cueva (Hospital Universitario Infanta Leonor); Isabel Belinchón (Hospital General Universitario de Alicante); Fran J. Gómez-García, Rafael Jiménez (Hospital Universitario Reina Sofía); Enrique Herrera-Ceballos, Enrique Herrera-Acosta (Hospital Universitario Virgen de la Victoria); José Luis López-Estebaranz, Diana Patricia Ruiz-Genao (Fundación Hospital de Alcorcón); Marta Ferrán Farrés (Hospital del Mar, Parc de Salut Mar de Barcelona); Mercè Alsina (Hospital Clinic de Barcelona); Ofelia Baniandrés, Lula Nieto (Hospital General Universitario Gregorio Marañón); José Luis Sánchez-Carazo (Hospital General Universitario de Valencia); Antonio Sahuquillo-Torralba, Rafael Botella-Estrada, Conrad Pujol Marco (Hospital Universitario La Fe de Valencia); Lourdes Rodríguez Fernández-Freire (Hospital Universitario Virgen del Rocío de Sevilla); Almudena Mateu Puchades (Hospital Universitario Dr. Peset), Ángeles Flórez Menéndez, Laura Salgado, Beatriz González Sixto (Complexo Hospitalario Universitario de Pontevedra); Noemí Eiris (Complejo Asistencial Universitario de León); Ignacio García-Doval, Miguel Ángel Descalzo Gallego, Marina de Vega Martínez (Fundación Piel Sana AEDV).
The BIOBADADERM project is promoted by the Fundación Piel Sana Academia Española de Dermatología y Venereología, which receives financial support from the Spanish Medicines and Health Products Agency (Agencia Española de Medicamentos y Productos Sanitarios) and from pharmaceutical companies (Abbott/Abbvie, Pfizer, MSD, Novartis, Lilly, Janssen and Almirall).
Collaborating pharmaceutical companies were not involved in the design and conduct of the study; collection, management, analysis and interpretation of data; preparation, review, or approval of the manuscript; decision to submit the manuscript for publication.
The authors are grateful to Paloma Hernández Fernández for copyediting the manuscript.
The patients in this manuscript provided written informed consent to publication of their case details.
Conflicts of interest. GC has been reimbursed by Janssen, Abbvie, Novartis, Pfizer, MSD and Celgene for advisory service and conference. RR acted as consultant and/or speaker for and/or participated in clinical trials as IP for Abbvie, Almirall, Celgene, Janssen, Leo Pharma, Lilly, Novartis, MSD and Pfizer-Wyeth. CF has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Amgen, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Merck Sharp & Dohme, Novartis Pfizer and Almirall. ED acted as consultant for Abbott, Amgen, Astellas, Centocor Ortho Biotech Inc, Galderma, Glaxo, Jansenn-Cilag, Leo Pharma, Novartis, Pfizer, MSD and Celgene, received honoraria form Abbott, Amgen, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, MSD, Celgene, participated in a speakers bureau for Abbott, Pfizer, MSD and Janssen and received grants from Pfizer, Abbott, Janssen and MSD. PdlC acted as a consultant and/or speaker for Janssen-Cilag, AbbVie, MSD, Pfizer, Novartis, Lilly, Almirall, UCB, Biogen, Celgene, Amgen, Sandoz, Sanofi and Leo-Pharma. IB acted as a consultant and/or speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including Janssen Pharmaceuticals Inc, Almirall SA, Lilly, AbbVie, Novartis, Celgene, Biogen Amgen, Leo-Pharma, Pfizer-Wyeth, and MSD. EH-A has served as consultant and/or speaker with Leo Pharma, Novartis, Janssen, Lilly, Celgene y Abbvie. DR-G has been reimbursed by Pfizer, Janssen, Celgene, Abbvie, Novartis and LeoPharma for advisory services and conferences. MF has participated as speaker and/or advisor for Janssen, Lilly, Novartis, Pfizer, MSD, Abbvie Celgene and Almirall. MA gave expert testimony for Merck-Schering Plough, Pfizer, Janssen, Novartis and Abbott. OB-R acted as a consultant and/or speaker for Janssen-Cilag, AbbVie, Pfizer, Novartis, Lilly, Celgene, Leo Pharma and Almirall. JLS-C participated as AB from Janssen, Novartis and Leo Pharma. AS-T has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis and Pfizer. LR acted as a consultant and speaker for Janssen-Cilag, AbbVie, MSD, Pfizer, Novartis, Lilly, Almirall, Celgene and Leo-Pharma. JV-A participated as AB from Janssen, Novartis, AbbVie, Almirall and Celgene. CG-D participated as AB from AbbVie, Almirall and speaker for Janssen, Lilly and Celgene.JMC has participated as speaker and/or advisor for Celgene, Janssen, Lilly, Novartis, Leo Pharma, Pfizer, MSD, Abbvie, Biogen Amgen. ML-V acted as a consultant and speaker and participated in clinical trials for Janssen-Cilag, AbbVie, Celgene, Pfizer, Novartis, Lilly, Almirall and Leo-Pharma. EH-C has served as a consultant and/or speaker for and/or participated in clinical trials as IP and sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Janssen-Cilag, LEO Pharma, Lilly, Novartis and Pfizer. JLL-E participated as AB and received educational grants from Janssen, Abbvie, MSD, Lilly, Novartis, LeoPharma, Pfizer. RB-E has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis and Pfizer. CP-M has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by companies that manufacture drugs used for the treatment of psoriasis, including AbbVie, Celgene, Janssen-Cilag, LEO Pharma, Lilly, Novartis and Pfizer. IG-D received travel grants for congresses from Abbvie, MSD and Pfizer.
REFERENCES
- Jahn I, Bornhorst C, Gunther F, Brand T. Examples of sex/gender sensitivity in epidemiological research: results of an evaluation of original articles published in JECH 2006-2014. Health Res Policy Syst 2017; 15: 11.
- Mazure CM, Jones DP. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health 2015; 15: 94.
- Takeshita J. Identifying Disparities in dermatology: the importance of measuring differences that matter. JAMA Dermatol 2018; 154: 1251–1253.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 205–212.
- Hagg D, Eriksson M, Sundstrom A, Schmitt-Egenolf M. The higher proportion of men with psoriasis treated with biologics may be explained by more severe disease in men. PLoS One 2013; 8: e63619.
- van der Schoot LS, van den Reek J, Groenewoud JMM, Otero ME, Njoo MD, Ossenkoppele PM, et al. Female patients are less satisfied with biological treatment for psoriasis and experience more side-effects than male patients: results from the prospective BioCAPTURE registry. J Eur Acad Dermatol Venereol 2019; 33: 1913–1920.
- Hagg D, Sundstrom A, Eriksson M, Schmitt-Egenolf M. Severity of psoriasis differs between men and women: a study of the clinical outcome measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol 2017; 18: 583–590.
- Regitz-Zagrosek V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep 2012; 13: 596–603.
- Bohm D, Stock Gissendanner S, Bangemann K, Snitjer I, Werfel T, Weyergraf A, et al. Perceived relationships between severity of psoriasis symptoms, gender, stigmatization and quality of life. J Eur Acad Dermatol Venereol 2013; 27: 220–226.
- Obradors M, Blanch C, Comellas M, Figueras M, Lizan L. Health-related quality of life in patients with psoriasis: a systematic review of the European literature. Qual Life Res 2016; 25: 2739–2754.
- Wojtyna E, Łakuta P, Marcinkiewicz K, Bergler-Czop B, Brzezińska-Wcisło L. Gender, Body image and social support: biopsychosocial deter-minants of depression among patients with psoriasis. Acta Derm Venereol 2017; 97: 91–97.
- Zweegers J, van den Reek JM, van de Kerkhof PC, Otero ME, Kuijpers AL, Koetsier MI, et al. Body mass index predicts discontinuation due to ineffectiveness and female sex predicts discontinuation due to side-effects in patients with psoriasis treated with adalimumab, etanercept or ustekinumab in daily practice: a prospective, comparative, long-term drug-survival study from the BioCAPTURE registry. Br J Dermatol 2016; 175: 340–347.
- Warren RB, Smith CH, Yiu ZZN, Ashcroft DM, Barker J, Burden AD, et al. Differential Drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015; 135: 2632–2640.
- Davila-Seijo P, Garcia-Doval I. Drug survival analysis is not a good method for assessing the safety or effectiveness of systemic therapies in psoriasis. Actas Dermosifiliogr 2017; 108: 3–5.
- Rivera R, Garcia-Doval I, Carretero G, Dauden E, Sanchez-Carazo J, Ferrandiz C, et al. [BIOBADADERM, the Spanish Registry of Adverse Events Associated with Biologic Drugs in Dermatology: first report]. Actas Dermosifiliogr 2011; 102: 132–141 (in Spanish).
- Davila-Seijo P, Dauden E, Descalzo MA, Carretero G, Carrascosa JM, Vanaclocha F, et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM registry. J Invest Dermatol 2017; 137: 313–321.
- D’Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011; 30: 377–399.
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991; 10: 585–598.
- Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017; 36: 4391–4400.
- Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 2019; 80: 251–265.e219.
- Lesuis N, Befrits R, Nyberg F, van Vollenhoven RF. Gender and the treatment of immune-mediated chronic inflammatory diseases: rheumatoid arthritis, inflammatory bowel disease and psoriasis: an observational study. BMC Med 2012; 10: 82.
- Fernandez-Torres RM, Pita-Fernandez S, Fonseca E. Quality of life and related factors in a cohort of plaque-type psoriasis patients in La Coruna, Spain. Int J Dermatol 2014; 53: e507–511.
- Maul JT, Navarini AA, Sommer R, Anzengruber F, Sorbe C, Mrowietz U, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. J Eur Acad Dermatol Venereol 2019; 33: 700–708.
- Warren RB, Marsden A, Tomenson B, Mason KJ, Soliman MM, Burden AD, et al. Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol 2019; 180: 1069–1076.
- Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013; 177: 292–298.
- Richter L, Vujic I, Sesti A, Monshi B, Sanlorenzo M, Posch C, et al. Etanercept, adalimumab, and ustekinumab in psoriasis: analysis of 209 treatment series in Austria. J Dtsch Dermatol Ges 2017; 15: 309–317.
- Pogácsás L, Borsi A, Takács P, Remenyik É, Kemény L, Kárpáti S, et al. Long-term drug survival and predictor analysis of the whole psoriatic patient population on biological therapy in Hungary. J Dermatol Treat 2017; 28: 635–641.
- Runnels V, Tudiver S, Doull M, Boscoe M. The challenges of including sex/gender analysis in systematic reviews: a qualitative survey. Syst Rev 2014; 3: 33.
- Di Cesare A, Bianchi C, Pescitelli L, Ricceri F, Rosi E, Pimpinelli N, et al. Risk of acute infections in psoriatic patients during biologic therapies is linked to gender. J Eur Acad Dermatol Venereol 2019; 33: e362–e364.
- Mourad A, Straube S, Armijo-Olivo S, Gniadecki R. Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol 2019; 181: 450–458.