Malignant eccrine porocarcinoma is a rare skin adnexal cancer arising from the sweat glands. Little is known about the epidemiology and incidence of eccrine porocarcinoma. This registry-based study examined the epidemiology and incidence data for eccrine porocarcinoma from the Finnish Cancer Registry. The study included all persons diagnosed with eccrine porocarcinoma in 2007 to 2017. There were 69 cases in the study period; 34 (49%) male and 35 (51%) female patients. Mean age at diagnosis was 75.5 years. Incidence for men was 0.06 per 100,000 person-years and for women 0.04 per 100,000 person-years adjusted for age according to the World Standard Population. Incidence increased with age. There was one eccrine porocarcinoma-specific death among the 69 patients. The incidence of eccrine porocarcinoma in Finland is therefore low. The mean age at time of diagnosis and the location of eccrine porocarcinoma are consistent with previous reports. The survival of patients with eccrine porocarcinoma is high.
Key words: eccrine porocarcinoma; incidence; survival.
Accepted Dec 7, 2020; Epub ahead of print Dec 14, 2020
Acta Derm Venereol 2021; 101: adv00363.
doi: 10.2340/00015555-3718
Corr: Virve Koljonen, Department of Plastic Surgery, University of Helsinki and Helsinki University Hospital, FIN-00029 Helsinki, Finland. E-mail: virve.koljonen@hus.fi
SIGNIFICANCE
Eccrine porocarcinoma is a skin cancer arising from skin sweat glands. Not much is known about the behaviour of eccrine porocarcinoma or its rate in populations. This study analysed data from the Finnish Cancer Registry, to determine the incidence of patients with eccrine porocarcinoma in Finland during 2007 to 2017. There were a total of 69 patients during this period, of whom half were women and half were men. The patients had a mean age of over 75 years. The incidence was 0.045 per 100,000 person-years. One patient died of eccrine porocarcinoma. Eccrine porocarcinoma is very rare skin cancer and the mortality is lower than previously reported.
INTRODUCTION
Malignant eccrine porocarcinoma (EPC) is a rare skin cancer, which arises from the intraepidermal part of the sweat gland, the acrosyringium. EPC occurs predominantly in elderly people;The reported mean age at diagnosis varied from 62 to 76 years (1–6). The first reported cases of EPC were described in 1963by Pinkus & Mehregan, who called it “epidermotropic eccrine carcinoma” due to its morphological similarities to benign poroma and to Paget’s disease (7). Mishima & Moriko later coined the term “eccrine porocarcinoma”, after studying oncogenic differentiation in sweat glands (8).
Published findings regarding the sex-specific incidence of EPC are contradictory. Systematic reviews of 453 cases and 206 cases show contradicting reports of the incidence between sexes (4, 9). A recent study of the data from the National Cancer Database in the USA showed slight male overrepresentation; 51.2% vs 48.8% (10). These systemic reviews and the National Cancer Database found that the most common locations of EPC are the head and neck area and lower limbs (4, 9, 10).
EPC commonly presents as a firm nodule or a plaque of violet or erythematous colour. EPC can also present as a skin-coloured plaque with oozing. Ulceration of the lesion may also occur (1, 9, 11). The median size of the tumour at diagnosis is reported to be 2 cm (2). The mean time between the appearance of the tumour and diagnosis has been reported as 5–9 years (2, 9), but may vary from 4 days to 60 years (1, 2, 5, 9).
EPC is difficult to diagnose; cutaneous squamous cell carcinoma is the most common misdiagnosis and, less frequently, other cutaneous cancers (1–3). It has been suggested that EPC arises from its benign counterpart, eccrine poroma. Robson et al. (2) observed that 18% of the EPC cases developed from a background of eccrine poroma, and Riera-Leal et al. (5) observed that 12% developed from this background. Shaw et al. (1) concluded that all 27 EPC cases in their study developed alongside a benign eccrine poroma.
The current study analysed data from the Finnish Cancer Registry (FCR) to determine the incidence of EPC in Finland during 2007 to 2017. As a secondary aim, this study also estimated the survival of patients with EPC.
PATIENTS AND METHODS
The institutional ethics committee of Helsinki University Hospital approved the study (HUS/358/2018). After consulting the data protection authority, the Finnish Institute of Health and Welfare granted permission to use the registry information on patients with EPC from the FCR in scientific research.
The FCR was founded in 1952 and maintains a nationwide population-based database of all cancer cases in Finland. Its coverage is virtually complete, as evidenced by clinical studies on national cancer incidence and treatment results (12). All physicians, hospitals and pathology and haematology laboratories in Finland are obliged to submit data on cancer patients to the FCR.
The FCR coding system was updated in 2007, from the 7th version of the International Classification of Diseases (ICD-7) to the International Classification of Diseases for Oncology, version 3 (ICD-O-3). Before 2007 there was no morphology code for EPC, and it was not possible to separate EPC as a separate disease entity. Therefore, this study focused on persons diagnosed during 2007 to 2017 with EPC (ICD-O-3 morphology code M8409/3).
Incidence rates per 100,000 person-years were calculated by sex and 5-year age groups, using population counts from Statistics Finland. The incidence rates were age-standardized to the World Standard Population (WSP) version 1966 (13). To compare these results with previous studies age-standardized incidence rates were also calculated, using the European Standard Population (ESP) (14).
For survival analyses, follow-up started from the date of diagnosis and ended at the date of death, emigration, or date of close of the study (31 December 2017), whichever occurred first.
RESULTS
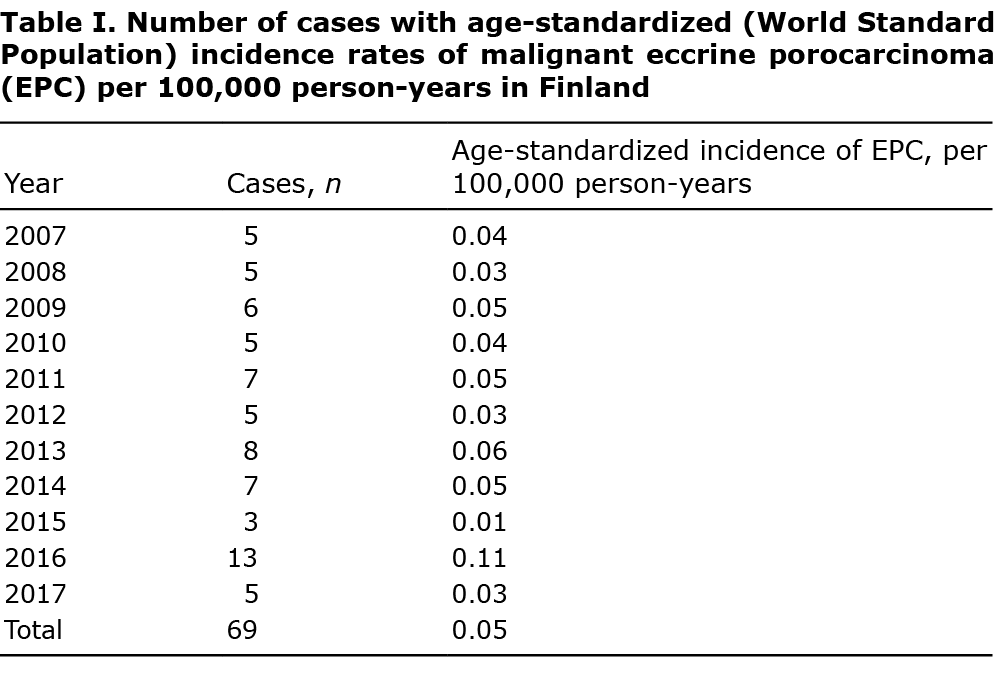
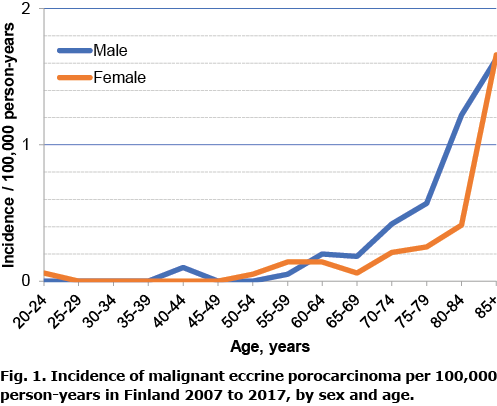
From 2007 to 2017 there were 69 cases of EPC recorded in the FCR. In all cases the diagnosis was confirmed by histology from the primary tumour. There were 34 (49%) male and 35 (51%) female patients and mean age at diagnosis was 75.5 years. Using the WSP, the age-adjusted incidence per 100,000 person-years was 0.06 for men and 0.04 for women, and, using the ESP, 0.15 and 0.10, respectively. The incidence rate was relatively consistent during the study period, except for the year 2016 (Table I). Incidence started increasing strongly after the age of 70 years (Fig. 1). In men incidence showed a small rise at a slightly earlier age than in women.
The most common location of EPC was on the lower limb, including the hip (20 cases; 29%) followed by unspecified parts of the face (16 cases; 23%) and the trunk, including the genitalia (12 cases; 17%). Other locations were the scalp and neck (9 cases; 13%), upper limb including the shoulder (7 cases; 10%), unspecified skin (3 cases; 4%), and in the ear and external auricular canal (2 cases; 3%). Taken together, all head and neck locations accounted for 39% of cases.
EPC was the first primary cancer in 42 of patients (61%) when basal cell carcinoma was included, and in 47 of patients (68%) when basal cell carcinoma was excluded. In one patient EPC and squamous cell carcinoma were diagnosed at the same time.
Survival of patients with EPC was similar to the general population of similar age (relative 5-year survival rate 104%). One EPC-specific death was recorded during the study period.
DISCUSSION
This study provides incidence estimates with a national coverage of the rare skin cancer EPC. There were approximately 7 cases of EPC annually between 2007 and 2017 in the Finnish population of 5.6 million. The mean age was consistent with previous studies; 75.5 years at presentation, respectively (4, 9). The age-adjusted incidence rate per 100,000 person-years was 0.06 for men and 0.04 for women when using WSP, and 0.15 for men and 0.10 for women using ESP. The locations of the primary tumour were consistent with previous reports (4, 9, 10); the head and neck region and lower limbs were the most common locations, as was observed in nearly 40% and 29%, respectively, of cases in the current study.
The largest series of EPC has been described by Robson et al. (2) (69 cases) and Goon et al. (15) (153 cases). Incidence rates of EPC have been rarely reported. A widely cited number is “0.01% of all skin cancers”, which refers to the study by Mehregan et al. (16). This number is derived from approximately one case of EPC per 13,000 specimens received in a dermatopathology laboratory. EPC represented 7% of the skin adnexal tumours in the Netherlands, and the age-standardized rate using the ESP for males in 1989–2010 was 0.04 and for females 0.03 per 100,000 person-years, i.e. approximately one-third of the rates observed in the current study (6). The age-standardized rates (ESP) from 2004 to 2013 from East England (males 2.4, females 1.3 per 100,000 person-years) were at least 10 times higher than our rates (15). In Minnesota, the incidence rates for both females and males were 0.2 per 100,000 person-years (17). They adjusted their rates to the US population from 2010, which is not very different from the ESP, and it can therefore be concluded that the incidence of EPC is only slightly higher than the rate in Finland. We strongly believe that the incidence rates found in the current study are true estimates of the numbers of cancer cases diagnosed as EPC in Finland, and that the number of EPCs misdiagnosed as other cutaneous cancers is small. Based on this information, it does not seem possible that the rates published from East England are correct. The rates predicted for year 2030 (15) would mean that the incidence of EPC would be approximately 30-fold that of the current rate in Finland or in Minnesota, and 100-fold that from a study in the Netherlands (6).
In contrast to earlier publications, EPC-specific mortality in the current series was very low, with only one death in 11 years. The survival of patients with EPC is therefore no worse than that of other Finns of similar age (5-year relative survival rate 104%). In the previous literature, at the time of diagnosis up to 30% of patients had metastases (4, 9), mostly in the regional lymph nodes. During follow-up of the EPCs, up to 25% progressed to regional metastases and 12% to distant metastases (1–3, 15). However, only 7–13% of patients died due to EPC (3, 16). In more recent reports, the aggressiveness of EPC was questioned, and the results suggest that aggressive behaviour and metastases may be an exception in EPC (3, 10, 18). The results of the current study are in line with the newer data; only one of the 69 patients with EPC died from EPC during the mean follow-up period of approximately 5 years.
This study found a relatively stable annual incidence of EPC, except for the year 2016, when it was much higher. However, in a rare cancer such as EPC, the possibility of chance cannot be excluded. Another Finnish study of a different rare skin cancer, Merkel cell carcinoma, observed that even one article or presentation at a congress may increase clinician’s knowledge and sensitivity to a rare skin cancer diagnosis and lead to a peak in incidence (19). The histological diagnosis of EPC is difficult. Diagnosis should be based on the presence of at least atypical poroid cells and ductal formation with typical histology. EPC has no specific immune-profile. EPC expresses carcinoembryonic antigen and epithelial membrane antigen, but so does benign poroma (2–4). Expression of CD117 can be used to differentiate EPC from squamous cell carcinoma, whereas S-100 can be used especially in diagnosis of dedifferentiated EPC and is expressed only in dendritic cells within the tumour (2, 13, 20–22). EPC may be erroneously diagnosed as squamous cell carcinoma and vice versa; however, the presence of neoplastic ducts is necessary for a diagnosis of EPC. Currently no immunostaining has been shown to have superiority for EPC diagnosis. As the data on EPC are accumulating, it is possible that a diagnostic immunostaining profile will be established.
Given the quality of the national cancer registry in Finland (12), the incidence estimates presented in this paper probably include most, if not all, diagnosed cases of EPC that occurred in Finland over the study period. However, it must be noted that this study cannot address the possibility of underdiagnosis of EPC. In particular, poorly differentiated EPC can easily be misdiagnosed as other skin carcinomas. The FCR gets information from pathology reports from all laboratories and, if there is conflicting information, selects the diagnosis considered most reliable. In earlier years FCR sometimes asked for pathological slides for re-evaluation by a panel of pathologists. The outcome of such quality control operations was that the quality of the pathological diagnoses performed in Finnish hospitals is good. In the case of EPC, it is possible that pathologists may have not been aware of this diagnosis option and therefore the number of cases of EPC is a minimum estimate of the true number.
In conclusion, this study confirms the low incidence of EPC in Finland. In line with recent studies, these results suggest very low mortality due to EPC. However, the rarity of EPC poses limitations on the interpretation of these results. This study was based solely on cancer registry data; further studies with clinical insight are warranted.
ACKNOWLEDGEMENTS
Ethics approval and consent to participate. An institutional ethics committee of Helsinki University Hospital approved the study plan. After consultation with the data protection authority, the National Institute of Health and Welfare granted permission to use the registry information on patients with EPC from the Finnish Cancer Registry (FCR) in scientific research. The study was performed in accordance with the Declaration of Helsinki.
Data availability. The Finnish Cancer Registry contributed data.
Funding. Jane and Aatos Erkko Foundation Grant.
The authors have no conflicts of interest to declare.
REFERENCES
- Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol 1982; 107: 675–680.
- Robson A, Greene J, Ansari N, Kim B, Seed PT, McKee PH, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol 2001; 25: 710–720.
- Belin E, Ezzedine K, Stanislas S, Lalanne N, Beylot-Barry M, Taieb A, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol 2011; 165: 985–989.
- Nazemi A, Higgins S, Swift R, In G, Miller K, Wysong A. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg 2018; 44: 1247–1261.
- Riera-Leal L, Guevara-Gutierrez E, Barrientos-Garcia JG, Madrigal-Kasem R, Briseno-Rodriguez G, Tlacuilo-Parra A. Eccrine porocarcinoma: epidemiologic and histopathologic characteristics. Int J Dermatol 2015; 54: 580–586.
- Stam H, Lohuis PJ, Zupan-Kajcovski B, Wouters MW, van der Hage JA, Visser O. Increasing incidence and survival of a rare skin cancer in the Netherlands. A population-based study of 2,220 cases of skin adnexal carcinoma. J Surg Oncol 2013; 107: 822–827.
- Pinkus H, Mehregan AH. Epidermotropic eccrine carcinoma. A case combining features of eccrine poroma and Paget’s dermatosis. Arch Dermatol 1963; 88: 597–606.
- Mishima Y, Morioka S. Oncogenic differentiation of the intraepidermal eccrine sweat duct: eccrine poroma, poroepithelioma and porocarcinoma. Dermatologica 1969; 138: 238–250.
- Salih AM, Kakamad FH, Essa RA, Rauf GM, S AM, H MS, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep 2017; 30: 13–16.
- Behbahani S, Malerba S, Karanfilian KM, Warren CJ, Alhatem A, Samie FH. Demographics and outcomes of eccrine porocarcinoma: results from the National Cancer Database. Br J Dermatol 2020; 183: 161–163.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol 2014; 53: 1053–1061.
- Pukkala E, Engholm G, Hojsgaard Schmidt LK, Storm H, Khan S, Lambe M, et al. Nordic Cancer Registries – an overview of their procedures and data comparability. Acta Oncol 2018; 57: 440–455.
- Segi M, Fujisaku S. Cancer mortality for selected sites in 24 countries (1950-1957): Department of Public Health, Tohoku University School of Medicine; 1960.
- Pace M, Lanzieri G, Glickman M, Zupanič T. Revision of the European Standard Population: report of Eurostat’s task force: Publications Office of the European Union; 2013.
- Goon PKC, Gurung P, Levell NJ, Subramanian P, Yong ASW, Lee KYC, et al. Eccrine porocarcinoma of the skin is rising in incidence in the East of England. Acta Derm Venereol 2018; 98: 991–992.
- Mehregan AH, Hashimoto K, Rahbari H. Eccrine adenocarcinoma. A clinicopathologic study of 35 cases. Arch Dermatol 1983; 119: 104–114.
- Tolkachjov SN, Schmitt AR, Muzic JG, Weaver AL, Baum CL. Incidence and clinical features of rare cutaneous malignancies in Olmsted County, Minnesota, 2000 to 2010. Dermatol Surg 2017; 43: 116–124.
- Avraham JB, Villines D, Maker VK, August C, Maker AV. Survival after resection of cutaneous adnexal carcinomas with eccrine differentiation: risk factors and trends in outcomes. J Surg Oncol 2013; 108: 57–62.
- Sahi H, Their J, Gissler M, Koljonen V. Merkel cell carcinoma treatment in Finland in 1986–2016 – a real-world data study. Cancers (Basel) 2020; 12: 1224.
- Goto K, Takai T, Fukumoto T, Anan T, Kimura T, Ansai S, et al. CD117 (KIT) is a useful immunohistochemical marker for differentiating porocarcinoma from squamous cell carcinoma. J Cutan Pathol 2016; 43: 219–226.
- Kurisu Y, Tsuji M, Yasuda E, Shibayama Y. A case of eccrine porocarcinoma: usefulness of immunostain for s-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol 2013; 25: 348–351.
- Nakazawa T, Kondo T, Sato E, Motosugi U, Niu D, Mochizuki K, et al. Subcutaneous porocarcinoma clinically presenting as a soft tissue tumor. J Cutan Pathol 2015; 42: 897–902.