ORIGINAL REPORT
Results of Mohs’ Micrographic Surgery of Periocular Basal Cell Carcinoma: The Swedish Experience
Kalliopi ERRIPI1, Daniel PAUSSEN1 and Karin SVEDBERG1,2
1Department of Ophthalmology, Sahlgrenska University Hospital, Region Västra Götaland, Mölndal, Sweden, 2Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
The Department of Ophthalmology, Sahlgrenska University Hospital, has until recently been the only eye clinic in the Nordic countries to perform Mohs’ micrographic surgery of basal cell carcinoma. This has led to the practice of only the most complicated basal cell carcinomas being operated on with this technique. The purpose of this study was to present the results of these surgeries in patients with at least 5 years of follow-up. A retrospective study of all patients operated upon in 2010–2015 was performed. Data were gathered from their medical charts. Primary outcome was recurrence of basal cell carcinoma. One-hundred and sixty-seven patients were operated on. Mohs’ micrographic surgery was used for tumours that were judged as highly aggressive on preoperative biopsy, had ill-defined borders, had recurred after previous surgery, or a combination of these factors. Nine recurrences (5.4% of all radical Mohs’ micrographic surgeries) were diagnosed after a mean postoperative time of 37 months (4–84 months). Interestingly, all of these 9 recurrences after Mohs’ micrographic surgery were in patients who had such surgery because of a recurrent basal cell carcinoma to start with. Good results can be achieved when operating on the most complicated periocular basal cell carcinomas with Mohs’ micrographic surgery but special care has to be taken to ensure radical borders when operating on recurring basal cell carcinomas.
Key words: basal cell carcinoma; eyelid; Mohs’ micrographic surgery; periocular area; recurrence; skin cancer.
SIGNIFICANCE
The most aggressive and complicated basal cell carcinomas in the eye region can successfully be operated on with Mohs’ micrographic surgery but special care has to be taken to ensure radical borders when operating tumours recurring after previous surgery.
Citation: Acta Derm Venereol 2024; 104: adv15765. DOI https://doi.org/10.2340/actadv.v104.15765.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
Submitted: Jun 30, 2023; Accepted: Mar 6, 2024; Published: Apr 2, 2024
Corr: Karin Svedberg, Department of Ophthalmology, Sahlgrenska University Hospital, Göteborgsvägen 31, SE-431 80 Mölndal, Sweden. E-mail: karin.svedberg@oft.gu.se
Competing interests and funding: The authors have no conflicts of interest to declare. The last-listed author has had a minor commission for SantenPharma AB.
This work has been carried out as part of our employments at the Sahlgenska University Hospital and no external funding sources have been needed.
INTRODUCTION
The most common skin cancer is the basal cell carcinoma (BCC) (1). Over 75% of BCCs occur in the head and neck region (2). Approximately 20% of BCCs appear in the periocular region (3). BCCs account for about 90% of all malignant eyelid tumours (4). The incidence of all BCCs is slowly increasing worldwide (3, 5) and in the Scandinavian countries (6). BCC is by far the most common form of cancer among the Swedish population (7).
The gold standard for the treatment of periocular BCC is surgical resection with oculoplastic reconstruction (8, 9). There are several tumour characteristics that are associated with higher recurrence rates, such as localisation in the periocular area, high infiltrative histopathological subtype, ill-defined clinical margins, recurrent lesions, or incompletely excised tumours (10). BCCs with a high risk of recurrence are preferably treated with Mohs’ micrographic surgery (MMS), which allows for a complete examination of all tissue margins, minimising the risk of recurrence and avoiding unnecessary removal of healthy tissue (7, 11).
MMS was developed by Frederic E. Mohs in the 1930s and has been used successfully in the treatment of malignant eyelid tumours (12). BCCs can be removed by excising successive layers and microscopically examining 100% of the peripheral and deep margins (4, 13, 14). Evaluation of 100% of the margin leads to the highest cure rates for BCC (15). Additionally, MMS contributes to the preservation of normal tissues (10, 16–18) and may spare vital structures on the eyelids, protecting the lacrimal system and canthi. What is most vital is that the eye is preserved. “Perhaps, in no other location are the dual benefits of MMS (high cure rate and tissue preservation) so important” (14).
At the time of surgery, our clinic was the only eye clinic in Scandinavia performing MMS, which led to a selection towards the inclusion of the most difficult cases in our material. Hence, our material has a different case mix from many other publications on the results of surgery according to Mohs. Our group has previously presented a study using a smaller base of material with shorter follow-up times (19, 20).
The aim of this study is to present the results from our investigations of MMS for BCCs in the periocular area in patients with at least 5 years of follow-up, with a focus on recurrences and postoperative complications. Additionally, we seek to encourage others to commence offering MMS for BCCs in the periocular area.
MATERIALS AND METHODS
A retrospective study with data collected from the medical charts of all the patients who underwent MMS because of BCC in the periocular area from 2010 and including 2015 at the Department of Ophthalmology, Sahlgrenska University Hospital, Sweden, was carried out. It was approved by the Swedish Ethical Review Authority (2019-02100) and was conducted in compliance with the ethical principles for medical research contained in the Declaration of Helsinki.
All patients had biopsy-verified BCC and MMS was reserved for individuals with the most complicated tumours (highly aggressive, recurring lesions, ill-defined tumours, or combinations of these). Patients were referred from different parts of Sweden.
All surgery was performed by an oculoplastic surgeon with the assistance of a dermatopathologist. The oculoplastic surgeon performed the surgical part of MMS (tumour excision and reconstructive surgery) whereas the dermatopathologist was responsible for the histopathological analysis of the specimens.
Data on BCC type, periocular location, side, year of surgery, age and gender of the patient, any known immunosuppression, number of resections to reach tumour-free margins, postoperative complications, recurrences, time to recurrence, and additional treatments were gathered from the medical records. For those patients who lived outside the Gothenburg region and were followed by their referring physician postoperatively, copies of medical charts were requested.
Follow-up was carried out for at least 5 years. For 152 patients (91%), follow-up with a clinical examination was carried out 1 year after MMS and then a follow-up of their medical record for at least 5 years. Copies of medical records were requested for those patients who lived outside the Gothenburg region and were followed by their referring physician postoperatively. Patients who presented signs of potential recurrence were referred to our department for additional clinical control.
In Sweden BCCs are classified histopathologically according to the system proposed by Jernbeck and Glaumann (21) (“Sabbatsbergsmodellen”) (Fig. 1). The following classification is used: nodular BCCs (Glas 1A), superficial BCCs (Glas 1B), infiltrative, moderately aggressive BCCs (Glas II), and highly aggressive, morpheiform BCCs (Glas III).
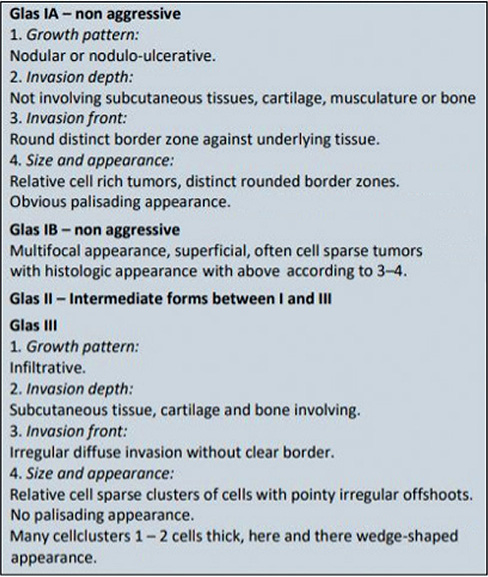
Fig. 1. Histopathological classification of basal cell carcinoma according to “Sabbatsbergsmodellen”.
Surgical methods
MMS was performed under local anaesthesia. The first Mohs’ excision was done with a clinical tumour margin of 2–3 mm. Long-acting bupivacaine 0.5% (Marcaine®) and lidocaine 1% (Xylocaine®) with adrenaline was used for infiltration anaesthesia. The marked tissue was excised in a single piece with a 45º angle incision. Bipolar diathermy was used for haemostasis. The excised tissue was then transferred to an adjacent laboratory where it was processed into horizontal sections of frozen tissue that theoretically allowed for inspection of 100% of the lateral and deep resection margins.
The histotechnician mapped the excised tissue on a piece of paper, indicating the source of the specimen and the color-coding. The specimen was inverted and several slides were taken from the bottom of the excised tissue and stained with haematoxylin and eosinophil. The slides were examined by a dermatopathologist to ascertain whether the margins were tumour-free. Areas with identified tumour were noted on the drawing and the procedure was repeated until all resected margins were free of BCC. Tumour free margins do not comprise an exact figure. We always have at least 3 tumour-free complete slides of uninflamed tissue, which amounts to approximately 0.24 mm (Fig. 2). Finally, reconstruction of the surgical defect was carried out by the oculoplastic surgeon.
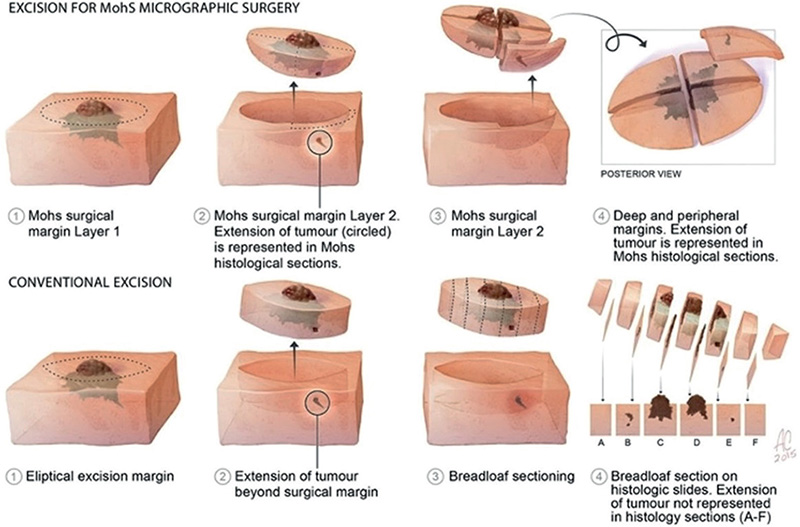
Fig. 2. Definition of Moh’s micrographic surgery. (Picture published as free utility on the internet, origin unknown.)
Statistical analysis
Statistical analyses were performed with SPSS version 25.0 (IBM Corp, Armonk, NY, USA). Descriptive statistics and Fisher’s exact test were applied when relevant. All tests were two-sided and a p-value of < 0.05 was considered significant.
RESULTS
A total of 167 patients were operated on during the study period. An example illustrated in Fig. 3. Demographic data, some information concerning the BCCs, and some surgical information are presented in Table I. A total of 116 patients (69.5%) had a primary BCC whereas for 51 (30.5%) the indication for performing an MMS was a recurring tumour. After at least 5 years of follow-up 9 recurrences (5.4%) were identified. These 9 patients are presented in Table II. All recurrences occurred in patients who were operated on with MMS because of recurring BCCs. This was statistically significant (p < 0.001, Fisher’s exact test).
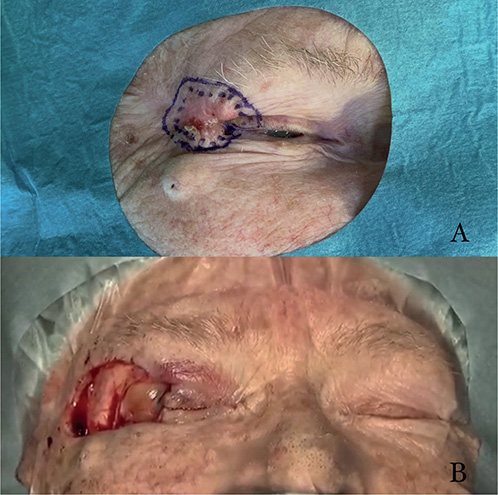
Fig. 3. Basal cell carcinoma of the eyelid. (A) Before excision with markings of clinical boundaries. (B) Surgical area after last excision and before reconstruction. Published with permission from the patient.
Sixty-two patients (37%) had some postoperative complication, presented in Table III. The most common of these was cicatricial ectropion.
Five patients (3%) required additional cryosurgery after MMS because of remaining superficial BCC at the edge of the resected area. It was impossible to achieve radical MMS for 12 individuals because of BCC ingrowth into the orbit or bone. These patients received postoperative radiotherapy as a supplement. None of the patients who needed postoperative additional cryotherapy or radiotherapy presented a recurrence.
There were 10 patients (6%) who passed away prior to the 5-year follow-up, and for 5 patients (3%) post-operative information was incomplete.
Two patients with recurrences were immunosuppressed. One patient had Hodgkin’s lymphoma and the other suffered from chronic kidney disease.
DISCUSSION
The recurrence rate during 5-year follow-up (5.4%) and the mean time to recurrence (36.5 months) found in this study were comparable to that found in previous studies (7). Other studies concerning periocular BCCs treated with MMS have shown similar outcomes and have emphasised the fact that BCCs in the periocular area are difficult to radically excise when not performing MMS (22–24).
In light of our specific difficult case mix our results must be considered acceptable. All BCCs were located periocularly, which is regarded as a high-risk area (10). Most of the BCCs included (n = 138, 83%) had an aggressive histopathological subtype (Glas type II or type III), ill-defined clinical margins, were recurrent BCCs, or had previously undergone an incomplete primary surgical excision or cryotherapy or a combination of those factors. These features are all known to carry a significant risk of recurrence (17). Those patients (n = 23) who were operated on with BCC Glas type I had either recurrent tumours, ill-defined margins, or a combination of these factors.
Other European centres have reported 5-year recurrence rates of 1.7–3.2% for primary BCCs and 4.8–6.7% for recurrent BCCs using the same MMS technique to treat BCCs in the facial area (18, 25, 26).
Although there were both primary BCCs (n = 116, 69.5%) and recurrent BCCs (n = 51, 30.5%) included in the study, recurrence after MMS occurred only in those patients who had been operated on because of recurrent BCCs. This was a surprising and interesting finding, which might indicate the importance of complete excision of all scar tissue to ensure that all tumour cells are eradicated.
Previously published studies report cases where the scar tissue is not completely excised and therefore the tumour is missed (27, 28). Other studies have tried to explain why recurrences can still occur after treatment with MMS, where theoretically 100% of the resection margins has been histopathologically examined (29–31). Such a factor could, for example, be incomplete Mohs’ slides (lacking part of the epidermis or dermis) so that the resection margins were not completely visible and the tumour could be missed (30). Special care has to be taken to ensure radical borders when operating on recurring BCCs.
An equally interesting outcome with almost 0% recurrence rate has been shown when performing MMS on primary BCCs, but other studies have shown as well (17) that there is an increased recurrence rate when performing MMS on recurrent BCCs (16). The complication rate and the type of complications are comparable to previous studies (32).
The most common complication was cicatricial ectropion (n = 19, 11.4%). This might be partially explained by the fact that the lower eyelid was the most common localisation of BCCs (n = 87, 52%).
The most common required reconstruction when the defect was in the lower eyelid was a full-thickness skin graft combined with surgical flaps. This may sometimes lead to a cicatricial ectropion if the postoperative care is not optimal. Lagophthalmos and epiphora were presented in 6% and 4.8% respectively. Both complications are also often associated with cicatricial ectropion.
Periocular complications from the surgical management of BCCs are best managed by preventing them in the first place with excellent surgical technique and preoperative planning.
Another interesting finding was that none of the patients who required additional radiotherapy after MMS presented a recurrence during this 5-year follow-up, despite the fact that these tumours were some of the most complicated BCCs. Radiotherapy as management of BCCs is an established therapy but there are few publications evaluating radiotherapy as an additive when tumour-free margins have not been achieved with MMS. However, Leshin et al. (33) have suggested that modern radiotherapy techniques may offer adequate tumour control with less damage to surrounding tissues.
Radiotherapy is often reserved for unresectable lesions or for patients unfit for surgery. It appears that radiotherapy is associated with a high rate of local control and a low rate of serious side effects (34). In general, adjuvant radiotherapy is often recommended after wide excision with narrow or positive margins or with high-risk factors present, including perineural invasion, invasion of the bone or nerves, or with recurrent disease (35). The goal of adjuvant radiotherapy is to further minimise the risk of local or regional recurrence. Orbital invasion is rare with a reported incidence of 2% and it can even lead to exenteration (36).
Follow-up one year after surgery and subsequent visits when needed might be considered a limitation in our study. We have made extensive efforts in collecting medical records for 5 years for all patients. As ours was the only eye clinic at the time to perform MMS we judge it very likely that any recurrence should have been reported to or referred to us.
In conclusion, for BCCs in the periocular region, MMS should be the treatment of choice for both primary high-risk BCCs and recurrent periocular BCCs. The increased recurrence rate, demonstrated by recurrent BCCs, highlights the need for comprehensive follow-up and special attention when operating on recurring tumours.
ACKNOWLEDGEMENTS
This work was presented as a poster at the ESOPRS yearly meeting in Nice, 15–17 September 2022.
IRB approval status: Approved by the Swedish Ethical Review Authority (2019–02100).
REFERENCES
- Gandhi SA, Kampp J. Skin cancer epidemiology, detection, and management. Med Clin North Am 2015; 99: 1323–1335.
- Iorio ML, Ter Louw RP, Kauffman CL, Davison SP. Evidence-based medicine: facial skin malignancy. Plast Reconstr Surg 2013; 132: 1631–1643.
- Furdova A, Kapitanova K, Kollarova A, Sekac J. Periocular basal cell carcinoma: clinical perspectives. Oncol Rev 2020; 14: 420.
- Moul DK, Chern PL, Shumaker PR, Zelac DE, Greenway HT. Mohs micrographic surgery for eyelid and periorbital skin cancer. Int Ophthalmol Clin 2009; 49: 111–127.
- Saleh GM, Desai P, Collin JR, Ives A, Jones T, Hussain B. Incidence of eyelid basal cell carcinoma in England: 2000–2010. Br J Ophthalmol 2017; 101: 209–212.
- Kappelin J, Green AC, Ingvar Å, Ahnlide I, Nielsen K. Incidence and trends of basal cell carcinoma in Sweden: a population-based registry study. Br J Dermatol 2022; 186: 963–969.
- Paoli J, Daryoni S, Wennberg AM, Mölne L, Gillstedt M, Miocic M, et al. 5-year recurrence rates of Mohs micrographic surgery for aggressive and recurrent facial basal cell carcinoma. Acta Derm Venereol 2011; 91: 689–693.
- Kakkassery V, Heindl LM. SOP Standardisiertes Vorgehen in der Diagnostik und Therapie des periokulären Basalzellkarzinoms. Klin Monbl Augenheilkd 2017 Oct 9 [Online ahead of print].
- Rokohl AC, Kopecky A, Guo Y, Kakkassery V, Mor JM, Loreck N, et al. Chirurgische Resektion mit ophthalmoplastischer Rekonstruktion. Goldstandard beim periokulären Basalzellkarzinom. Ophthalmologe 2020; 117: 95–105.
- Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008; 159: 35–48.
- Drake LA, Dinehart SM, Goltz RW, Graham GF, Hordinsky MK, Lewis CW, et al. Guidelines of care for Mohs micrographic surgery. J Am Acad Dermatol 1995; 33: 271–278.
- Mohs FE. Micrographic surgery for the microscopically controlled excision of eyelid cancers. Arch Ophthalmol 1986; 104: 901–909.
- Treacy MP, Wynne NC, Gale JL, Duignan E, Moran B, Flynn AM, et al. Mohs micrographic surgery for periocular skin tumours in Ireland. Ir J Med Sci 2016; 185: 779–783.
- Monheit G, Hrynewycz K. Mohs surgery for periocular tumors. Dermatol Surg 2019; 45 Suppl 2: S70–s78.
- Wong E, Axibal E, Brown M. Mohs micrographic surgery. Facial Plast Surg Clin North Am 2019; 27: 15–34.
- Sin CW, Barua A, Cook A. Recurrence rates of periocular basal cell carcinoma following Mohs micrographic surgery: a retrospective study. Int J Dermatol 2016; 55: 1044–1047.
- Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database, part II: periocular basal cell carcinoma outcome at 5-year follow-up. Ophthalmology 2004; 111: 631–636.
- Smeets NW, Kuijpers DI, Nelemans P, Ostertag JU, Verhaegh ME, Krekels GA, et al. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face: results of a retrospective study and review of the literature. Br J Dermatol 2004; 151: 141–147.
- Lindgren G, Lindblom B, Bratel AT, Mölne L, Larkö O. Mohs’ micrographic surgery for basal cell carcinomas on the eyelids and medial canthal area. I. Characteristics of the tumours and details of the procedure. Acta Ophthalmol Scand 2000; 78: 425–429.
- Lindgren G, Lindblom B, Larkö O. Mohs’ micrographic surgery for basal cell carcinomas on the eyelids and medial canthal area. II. Reconstruction and follow-up. Acta Ophthalmol Scand 2000; 78: 430–436.
- Jernbeck J, Glaumann B, Glas JE. [Basal cell carcinoma: clinical evaluation of the histological grading of aggressive types of cancer]. Lakartidningen 1988; 85: 3467–3470 (in Swedish).
- Jebodhsingh KN, Calafati J, Farrokhyar F, Harvey JT. Recurrence rates of basal cell carcinoma of the periocular skin: what to do with patients who have positive margins after resection. Can J Ophthalmol 2012; 47: 181–184.
- Weesie F, Naus NC, Vasilic D, Hollestein LM, van den Bos RR, Wakkee M. Recurrence of periocular basal cell carcinoma and squamous cell carcinoma after Mohs micrographic surgery: a retrospective cohort study. Br J Dermatol 2019; 180: 1176–1182.
- Barnes EA, Dickinson AJ, Langtry JA, Lawrence CM. The role of Mohs excision in periocular basal cell carcinoma. Br J Ophthalmol 2006; 90: 660–661.
- Julian CG, Bowers PW. A prospective study of Mohs’ micrographic surgery in two English centres. Br J Dermatol 1997; 136: 515–518.
- Wennberg AM, Larkö O, Stenquist B. Five-year results of Mohs’ micrographic surgery for aggressive facial basal cell carcinoma in Sweden. Acta Derm Venereol 1999; 79: 370–372.
- Eliezri YD, Cohen PR. Cancer recurrence following Mohs micrographic surgery: a mechanism of tumor persistence. Plast Reconstr Surg 1992; 90: 121–125.
- Dzubow LM. False-negative tumor-free margins following Mohs surgery. J Dermatol Surg Oncol 1988; 14: 600–602.
- Smeets NW, Stavast-Kooy AJ, Krekels GA, Daemen MJ, Neumann HA. Adjuvant cytokeratin staining in Mohs micrographic surgery for basal cell carcinoma. Dermatol Surg 2003; 29: 375–377.
- Hruza GJ. Mohs micrographic surgery local recurrences. J Dermatol Surg Oncol 1994; 20: 573–577.
- Rapini RP. Pitfalls of Mohs micrographic surgery. J Am Acad Dermatol 1990; 22: 681–686.
- Carniciu AL, Jovanovic N, Kahana A. Eyelid complications associated with surgery for periocular cutaneous malignancies. Facial Plast Surg 2020; 36: 166–175.
- Leshin B, Yeatts P, Anscher M, Montano G, Dutton JJ. Management of periocular basal cell carcinoma: Mohs’ micrographic surgery versus radiotherapy. Surv Ophthalmol 1993; 38: 193–212.
- Cho M, Gordon L, Rembielak A, Woo TC. Utility of radiotherapy for treatment of basal cell carcinoma: a review. Br J Dermatol 2014; 171: 968–973.
- Strom T, Harrison LB. Radiotherapy for management of basal and squamous cell carcinoma. Curr Probl Cancer 2015; 39: 237–247.
- Sun MT, Wu A, Figueira E, Huilgol S, Selva D. Management of periorbital basal cell carcinoma with orbital invasion. Future Oncol 2015; 11: 3003–3010.