SHORT COMMUNICATION
Characterization of Paediatric Prurigo Nodularis: A Multicentre Retrospective, Observational Study
Rotem KYVAYKO1#, Tahel FACHLER-SHARP2#, Shoshana GREENBERGER3,4, Amir HOREV5§ and Vered MOLHO-PESSACH2§*
1Hebrew University of Jerusalem, Faculty of Medicine, Jerusalem, 2Department of Dermatology, Hadassah Medical Center, Hebrew University of Jerusalem, Faculty of Medicine, Jerusalem, 3Department of Dermatology, Sheba Medical Center, Tel Hashomer, 4Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv and 5Pediatric Dermatology Service, Soroka University Medical Center, Ben-Gurion University of the Negev, Beer-Sheva, Israel. *E-mail: rverem@hadassah.org.il
#These authors contributed equally to this study, and should be considered first authors. §These authors contributed equally to this study, and should be considered last authors.
Citation: Acta Derm Venereol 2024; 104: adv15771. DOI: https://doi.org/10.2340/actadv.v104.15771.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Jul 1, 2023. Accepted after review: Dec 12, 2023. Published: Feb 1, 2024
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Prurigo nodularis (PN) is a chronic inflammatory skin disorder, characterized by intensely pruritic, hyperpigmented, hyperkeratotic and symmetrically distributed nodules, that profoundly impairs patients’ quality of life (1). An itch-scratch vicious cycle results in disease exacerbation (2).
Epidemiologically, PN has an estimated prevalence in US adults of 72:100,000 (1). It is more common in middle-aged adults; patients with skin of colour are at increased risk. PN in adults is associated with increased rates of various skin disorders; mainly atopic dermatitis (AD), mental health disorders, endocrine, cardiovascular, renal disorders, and malignancies (mostly haematological) (1).
To date, there is a lack of data regarding paediatric PN including demographic and epidemiological features, clinical manifestations, treatments and response to treatments. A recent study showed association between paediatric PN and comorbidity, such as anxiety, attention deficit hyperactivity disorder (ADHD), AD and obesity. Children with PN appeared more likely than adults to have AD compared with their matched peers (odds ratio (OR) 37.7 vs 9.48) (3).
The aim of this study is to investigate the demographic and clinical features of paediatric PN.
MATERIALS, METHODS AND RESULTS
Children aged 0–18 years clinically diagnosed with PN were identified from databases of paediatric dermatology clinics of 3 tertiary medical centers in Israel: Hadassah Medical Center, Sheba Medical Center and Soroka Medical Center, from January 2008 to February 2021. Demographic and epidemiological features (sex, ethnicity, age), clinical manifestations of prurigo nodularis (age of onset, duration of disease, lesions’ locations), laboratory evaluation and data regarding treatments and response to treatments were extracted from medical records. Response to the various treatments was determined according to the description in the patients’ medical records and according to the need to pursue other treatments. T-tests, χ2 analyses and Fisher’s exact test were conducted to evaluate differences; p < 0.05 was considered statistically significant. This study was approved by the local Institutional Review Board (IRB) committees.
Demographics of the 66 identified children are shown in Table I; 40.9% were male, 71.2% were Jewish. Mean age at first evaluation was 8.9 years.
The medical background of the patients was collected. Comorbid diseases are shown in Table II. Atopic background (including asthma, allergic rhinitis, food/other allergies, and AD) was observed in 66.7% of patients, while 62.1% had AD. Familial atopic background was identified in 27.3%, while 13.6% had familial AD.
Mean follow-up (data available in 66 patients) was 2.65 years, with a median of 0.96 years (interquartile range (IQR) 3.3 years) Data regarding the duration of PN were available in 42 patients, with a mean of 2.88 years and median of 1.35 years (IQR 3.34).
Affected body regions are illustrated in Fig. 1, with the lower and upper extremities most affected (90.9%, 72.2%, respectively). Lesions on the face were found in 27.3% of patients. Patients with facial lesions were compared with patients without facial lesions regarding demographic and clinical characteristics. Patients with facial lesions had significantly higher rates of involvement of the back (61.1% vs 31.3%, p = 0.027), feet (27.8% vs 4.2%, p = 0.014) and hands (33.3% vs 8.3%, p = 0.02). Patients with facial lesions also had earlier age of disease onset (mean of 6.98 vs 9.47, p = 0.044) and higher number of affected sites (excluding facial) (mean of 3.9 vs 2.6, p = 0.015).
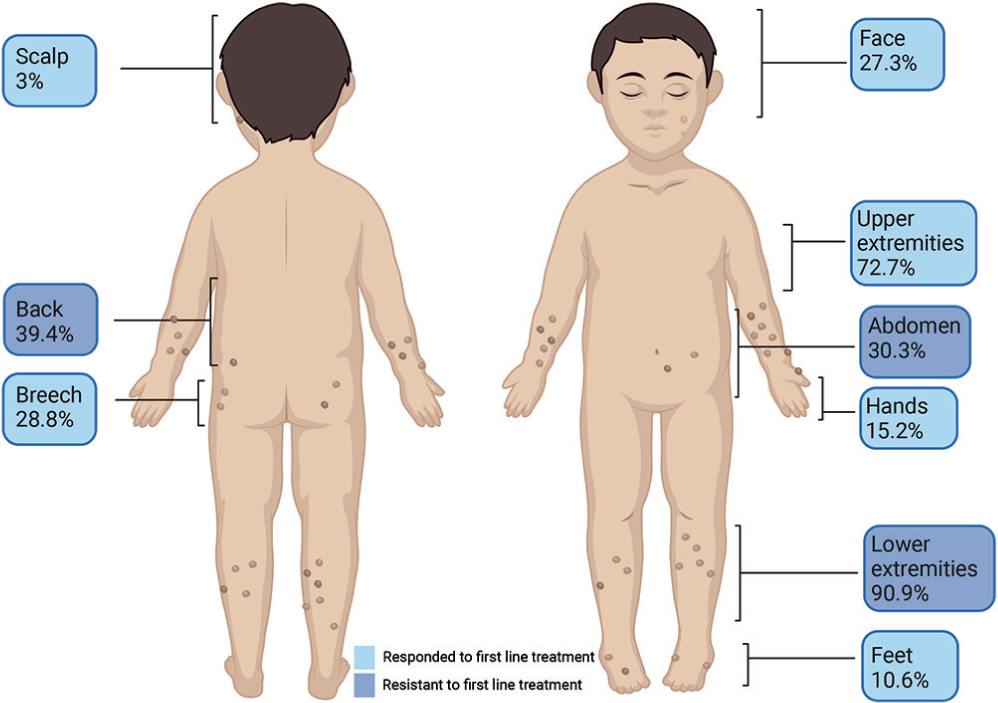
Fig. 1. Affected body regions in children with prurigo nodularis. Affected body regions in 66 patients with paediatric prurigo nodularis are presented. First-line treatment included: moisturizers, topical corticosteroids, topical calcineurin inhibitor and antihistamines. Second-line treatments included: narrowband ultraviolet B, ultraviolet A1, psychological therapy, intralesional corticosteroids, methotrexate, cyclosporine, systemic corticosteroids and dupilumab. Second-line treatments were given following incomplete/no response to first-line treatments.
Treatments given are shown in Table III. First-line treatments were defined as given at initial evaluation and included topical agents (moisturizers, topical corticosteroids and topical calcineurin inhibitors) and antihistamines. Response to treatment was considered “partial” when a partial response was mentioned in the medical records and when other treatments were needed following or in addition to the given treatment. More than 90% of patients were treated with topical corticosteroids with complete response rate of 11.7% and partial response rate of 63.3%. Other treatments were considered second line and were given following incomplete/no response to first-line treatments. Second-line treatments were applied in 60.6% of patients. Phototherapy was given in 56% of patients (mostly narrowband ultraviolet B; NBUVB), with an overall response rate of 72.9%. Systemic immunosuppressants (methotrexate and cyclosporine) were given in 15.2% (n = 10) with overall response rate of 66.7%. Methotrexate was used in 10.6%, cyclosporine in 7.6%. Dupilumab was given in 10.6% of patients. Out of the patients receiving methotrexate, cyclosporine and dupilumab, 85.7%, 80% and 85.7%, respectively had also AD. The mean number of different treatments per patient was 3.78 (median 4.00, IQR = 2).
| Treatment | Patients treated n (%)a | Response, n (%)b | |||
| No | Partial | Complete | Missing follow-upc | ||
| First-line | |||||
| Moisturizers | 44 (66.7) | 5 (11.4) | 34 (77.3) | 2 (4.5) | 3 (6.8) |
| Topical corticosteroids | 60 (90.9) | 6(10) | 38 (63.3) | 7 (11.7) | 9 (15.0) |
| Topical calcineurin inhibitor | 16 (24.2) | 1 (6.3) | 12 (75.0) | 1 (6.3) | 2 (12.5) |
| Antihistamines | 47 (71.2) | 8 (17.0) | 32 (68.1) | 2 (4.3) | 5 (10.6) |
| Second-line | |||||
| NBUVBd | 37 (56.1) | 6 (16.2) | 11 (29.7) | 16 (43.2) | 4 (10.8) |
| UVA1 | 2 (3) | 1 (50.0) | 1 (50.0) | 0 (0) | 0 (0) |
| Psychological therapy | 12 (18.2) | 0 (0) | 5 (41.7) | 0 (0) | 7 (58.3) |
| Intralesional corticosteroids | 5 (7.6) | 1 (20.0) | 3 (60.0) | 0 (0) | 1 (20.0) |
| Methotrexatee | 7 (10.6) | 1 (14.3) | 3 (42.9) | 0 (0) | 3 (42.9) |
| Cyclosporine | 5 (7.6) | 0 (0) | 4 (80.0) | 1 (20.0) | 0 (0) |
| Systemic corticosteroidse | 4 (6.1) | 0 (0) | 3 (75.0) | 0 (0) | 1 (25.0) |
| Dupilumabf | 7 (10.6) | 0 (0) | 2 (28.6) | 2 (28.6) | 3 (42.9) |
| Given treatments (n), mean ± SD | 3.78 ± 1.73 | ||||
| Median (CI) | 4.00 (1–9) | ||||
| aPercentage of the study population (total 66 patients). bPercentage of the population that received the specific treatment. cTreatment was recommended by dermatologist without further follow-up or response documentation. Adverse effects were documented in 8 patients: dDizziness, burns, burning sensation. eNausea. fBlurred vision, xerophthalmia. NBUVB: narrowband UVB; CI: confidence interval. |
|||||
Patients with PN and AD (AD-related PN) were compared with PN patients without AD. The number of given treatments was significantly higher in patients with AD-related PN (mean 4.32 vs 2.88, respectively, p = 0.001) and in patients with atopic background (mean 4.3 vs 2.7, p = 0.0005). Patients with AD-related PN were significantly more resistant to first-line treatments compared with patients without AD (73.2% vs 40.0%, respectively, p = 0.007). Patients with atopic background were also more resistant to first-line treatment compared with non-atopic patients (73.3% vs 33.3%, p = 0.002). Specifically, treatment with NBUVB was more common in AD-related PN than in patients without AD (68.3% vs 36.0%, respectively, p = 0.01).
No statistically significant associations were found between AD-related PN and demographic (sex, ethnicity), comorbidity, laboratory parameters, location of lesions, and number of affected sites.
Patients with lesions on the trunk and buttocks were more resistant to first-line treatments than patients without involvement of these sites; 84.6% vs 45.0% for back lesions (p = 0.001), 80.0% vs 52.2% for abdominal lesions (p = 0.033) and 84.2% vs 51.1% for buttock lesions (p = 0.013). Patients who were resistant to first-line treatment had a higher number of affected sites than patient who responded to first-line treatment (mean 3.73 vs 2.44, p = 0.005).
DISCUSSION
There is scarce data on paediatric PN. To the best of our knowledge, this study is the first to demographically and clinically characterize paediatric PN. A previous study on paediatric PN focused on the prevalence of paediatric PN and its associated comorbidity, but not on clinical characteristics (4). A higher prevalence of AD was found in patients with PN (62.1%) in children, compared with the prevalence observed in the adult population in the literature (up to 46.3%) (5) (p = 0.01). The above-mentioned study on paediatric PN similarly found a higher likelihood of having AD in children than adults (4). These findings could be explained by the higher rate of AD in the general paediatric population compared with in adults (6). The possibility of PN mistakenly diagnosed as AD, should also be considered.
The current study examined demographic and clinical features to assess the differences between AD-related PN and non-AD-related PN. A statistically significant association was shown between AD-related PN and resistance to first-line treatment.
Patient with AD-related PN received more second-line treatments than the non-AD-related patients. Higher rates of second-line treatments in AD-related PN could be explained by overlap in treatments of both diseases.
Regardless of AD-related PN, this study also found statistically significant associations between higher number of affected sites, truncal and buttocks’ lesions and resistance to first-line treatments.
Facial lesions were observed in 27.3% of patients in the childhood cohort. A survey performed among adult patients with PN reported facial involvement in 26% of patients, in accordance with the current findings (8). A statistically significant association was found between facial lesions and involvement of the back, hands and feet, earlier age of PN onset and a higher number of affected sites (excluding facial), suggesting facial lesions may be associated with a more severe disease course.
As mentioned above, previous studies in adults and 1 study in children found an association between PN and various comorbid diseases other than AD, such as psoriasis, affective disorders and endocrine disorders. Several comorbid diseases were observed in the current study cohort. However, due to a small sample size and lack of a control group, this study could not identify statistically significant associations between PN and any other comorbidity.
Although high response rates to several treatments were found, a complete response to first- and second-line treatments was uncommon, and multiple different treatments were needed, proving the persistent and resistant nature of paediatric PN.
This study is limited by its small sample size and retrospective nature. Data were collected from 3 tertiary centres and therefore may over-represent severe cases. Duration of treatments and duration of response to treatments were not recorded systematically in the medical records, and therefore data on this matter is lacking.
Larger population and prospective studies are required to enable further characterization of paediatric PN.
ACKNOWLEDGEMENTS
The authors thank Tali Bdolah-Abram from the Hebrew University of Jerusalem for her assistance with the statistical analysis. The study was conducted as part of the requirements for Rotem Kyvayko’s MD certificate at the Faculty of Medicine, the Hebrew University of Jerusalem.
Data are available on request, based on privacy or other restrictions. For requests, please contact the corresponding author, Vered Molho-Pessach.
This study protocol was reviewed and approved by the local Ethics Committee of each medical centre. Hadassah Medical Center, approval number [0182-21-HMO]. Soroka Medical Center, approval number [0304-21-SOR]. Tel Hashomer Medical Center, approval number [8239-21-SMC].
REFERENCES
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol 2020; 83: 1559–1565.
- Wong LS, Yen YT. Chronic nodular prurigo: an update on the pathogenesis and treatment. Int J Mol Sci 2022; 23: 12390.
- Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol 2020; 83: 1567–1575.
- Huang AH, Roh YS, Sutaria N, Choi J, Williams KA, Pritchard T, et al. Real-world disease burden and comorbidities of pediatric prurigo nodularis. J Am Acad Dermatol 2022; 86: 655–657.
- Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol 2013; 27: 550–557.
- Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol 2019; 80: 390–401.
- Aggarwal P, Choi J, Sutaria N, Roh YS, Wongvibulsin S, Williams KA, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol 2021; 46: 1277–1284.