Attention is known to modulate itch intensity. In contrast, the reverse relationship, i.e. the degree to which the presence of an acute itch affects attention, is currently not well understood. The aims of this study were to investigate whether acute itch induces an attentional bias towards or away from visual itch-related stimuli, and if so, whether it occurs in the early or later stages of processing. A volunteer sample of 60 healthy individuals were subjected to a skin prick (either histamine or placebo), followed by completion of a spatial cueing paradigm using itch-related and neutral words as cues, in order to obtain reaction time estimates of attentional bias. The results suggest that experience of acute itch induces attentional avoidance of visual itch threats. This attentional avoidance occurs at a later processing stage in the form of facilitated disengagement of attention from itch and/or delayed disengagement from neutral information.
Key words: attention; pruritus; agonist; histaminergic.
Accepted Mar 31, 2022; Epub ahead of print Mar 31, 2022
Acta Derm Venereol 2022; 102: adv00691.
DOI: 10.2340/actadv.v102.1626
Corr: Henning Holle, Department of Psychology, University of Hull, HU6 7RX Hull, UK. E-mail: h.holle@hull.ac.uk
SIGNIFICANCE
Attention has previously been demonstrated to modulate itch intensity, but the reverse relationship of how the presence of an acute itch modulates attention is currently not well understood. By experimentally manipulating acute itch in healthy volunteers, this study demonstrates, for the first time, that acute itch induces attentional avoidance of itch-related visual information. This attentional avoidance takes places in the later stages of processing, in the form of facilitated disengagement from a threat stimulus and/or delayed disengagement from neutral stimuli. Such attentional avoidance of itch threats can be risky, since it may be associated with accidental itch exacerbation in patients (e.g. when attentional avoidance results in failing to spot an itch-inducing substance).
INTRODUCTION
Itch is a universally experienced somatosensory sensation characterized by the urge to scratch. It is impossible to understand how itch is maintained, modulated and reacted to without considering the influence of attention. For example, itch may indicate the presence of a potentially harmful event, such as a stinging or biting insect (1), and attention has been shown to play a role in the experience and processing of acute itch (2). Much less well understood, however, is how the presence of an acute physical itch modulates attention to visual itch threats. There are 3 possibilities here. First, acute itch could lead to enhanced attentional processing of visual itch threats (i.e. hypervigilance); secondly, it could lead to attentional avoidance of visual itch threats; or thirdly, acute itch could have no impact on visual attention, similar to what has been reported for acute pain (3).
To the best of our knowledge, there is currently only one study that has experimentally manipulated acute itch to study its effect on visual attention (4). In that study, participants were asked to detect lateralized visual targets via button press with either the left or right index finger. In half of the blocks, an electrical itch-inducing stimulation was applied to one forearm. The results showed a response-slowing for the arm affected by acute itch, interpreted by the authors as participants disengaging attention away from the itch location. However, the effect was only statistically significant in the second half of the blocks, and the finding was not replicated in a later study (5). Furthermore, due to the nature of their design, the results do not allow conclusions about whether acute itch induces a specific attentional avoidance of visual itch threats, or whether it leads to generalized attentional avoidance.
According to Posner (6), visual attention can be conceptualized as a spotlight, in that attention focuses on 1 particular field at a time (7). The spotlight can shift to a different location with (i.e. overt attention) or without (i.e. covert attention) any physical eye movement (8). Control of the attentional spotlight is assumed to consist of 3 separate elements; initial orienting of attention, engagement with a stimulus, and subsequent disengagement from the stimulus (9). Attentional bias (AB) is the concept that some information types attract the attentional spotlight more strongly than others.
AB is often assessed by means of computerized reaction time tasks, such as the dot-probe task, the Stroop task, or the emotional spatial cueing task (10). For example, van Laarhoven et al. first induced acute itch in healthy volunteers, who then completed a Stroop and a dot-probe task (4). Participants showed a hypervigilant response pattern towards itch-related words and pictures in these tasks. However, this pattern cannot be causally attributed to the prior experience of acute itch, because of the absence of a no-itch control group in the dot-probe and Stroop task.
The current study therefore had 3 aims. First, to determine whether there is a causal relationship between acute itch and AB for visual itch-related information. Secondly, to determine the direction of that bias (hypervigilance, avoidance, or no bias) and thirdly, to assess whether the bias occurs early during the attentional engagement phase, or later during the disengagement phase. Participants underwent a skin prick (either histamine or placebo) and then performed a spatial cueing task to assess their AB.
MATERIALS AND METHODS
Participants
Sixty participants (54 female, 6 male, age range 18–37 years, mean ± standard deviation (SD) 20.35 ± 3.64 years) participated in the study, having given written informed consent. Data were collected between October 2018 and October 2019. Participants were randomly allocated to 1 of 2 groups (histamine, n = 29, or placebo, n = 31, unequal group sizes due to an administrative error). This sample size was chosen because it is sufficient to detect a large effect (Cohen’s d ≥ 0.8) in a between-group design with a probability of 80% (2-tailed test, α=0.05), as indicated by a priori power analysis (11). Participants were given either £8 or course credit for participation. The study protocol was approved by the local ethics committee.
Exclusion criteria were: history of allergy, acute or chronic skin conditions, injury to wrist area, vascular disease, low blood pressure, asthma, histamine intolerance, sensitive skin, hypersensitivity to certain food types, history of fainting during medical procedures, severe hearing or sight impairment, or pregnancy.
Emotional spatial cueing task
AB was measured with the emotional spatial cueing task (12), which is an adaptation of Posner’s spatial cueing task. A cue word on either the left- or right-hand side of a fixation cross was followed by a target that appeared either in the same location as the cue (so-called valid trial, 75% of trials) or on the opposite side of the screen (so-called invalid trial, 25% of trials). The participants’ task was to indicate via button press, as quickly and accurately as possible, on which side of the screen the target appeared. The keys “F” and “J” were used as response buttons, and participants were asked to use their left and right index fingers to make a response. The participant’s head was supported by a chin rest at a distance of 50 cm from the screen.
Each trial began with a fixation cross in the centre of the screen, along with 2 rectangles on either side (height=86 mm, width=58 mm, distance between rectangles=58 mm). After 1,000 ms, the cue word appeared in 1 of the rectangles for a duration of 200 ms. The cue then disappeared and after 50 ms the circular target (visual angle 0.92º) was presented (see Fig. 1). Thus, the stimulus onset asynchrony (SOA) between cue word onset and target was 250 ms.

The threat words used in the cueing task related to the sensory experience of itch and the visual appearance of itching skin. These words were previously shown to be related to the experience of histamine-induced itch (13). Control words were not related to itch, and were matched with threat words on a pair-wise basis for grammatical class, word length and lexical frequency using the subtlex-UK database (14) (see Table SI for stimuli).
Each block contained 32 trials, with 24 valid trials (12 neutral, 12 threat) and 8 invalid trials (4 neutral, 4 threat) and each participant completed 3 blocks. The experimental blocks were balanced with respect to the side on which a cue word appeared (left or right) and the experimental factors of Validity (valid vs invalid) and Cue type (threat vs neutral). The experiment was generated via Presentation Version 20.2 (www.neurobs.com).
Reaction times (RTs) are typically faster for valid trials, due to attention already being engaged with the cued location, and slower for invalid trials, reflecting the cost of reorienting attention to the un-cued location. The latency difference between invalid and valid trials is referred to as the validity effect (RTinvalid – RTvalid). Larger validity effects for threat trials than for neutral trials indicate an AB toward threat-related information (10) (hypervigilance). The opposite pattern (larger validity effects for neutral trials) indicates an AB away from threat-related information (avoidance).
Planned contrasts between valid and invalid trials for each cue type can be used to reveal whether AB occurs either at an early phase of attentional engagement or a later stage of attentional disengagement (10). AB effects at the early engagement phase are indicated by differences between neutral valid and threat valid trials, with a pattern of RTneutral_valid > RTthreat_valid signifying early hypervigilance (enhanced attentional capture), and a pattern of RTneutral_valid < RTthreat_valid reflecting early avoidance (delayed attentional capture). AB effects at the later disengagement phase are indicated by differences between neutral invalid and threat invalid trials, with a pattern of RTthreat_invalid > RTneutral_invalid indicating hypervigilance in the later stages of processing (difficulty to disengage/stronger attentional holding) and a pattern of RTthreat_invalid < RTneutral_invalid reflecting avoidance in the later stages of processing (facilitated attending away from threat).
Procedure
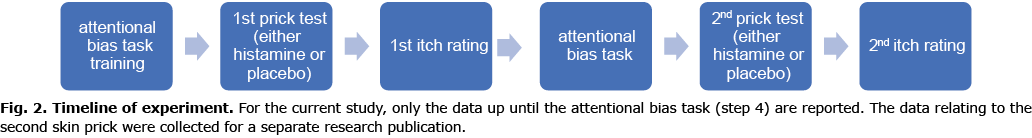
First, participants practiced a few trials of the spatial cueing task and familiarized themselves with the itch rating scale. Next, they received a prick test on 1 wrist, followed by itch ratings and completion of the spatial cueing task. This was followed by a second prick test on the opposite wrist, followed by a second itch rating exercise. One of the prick tests was a histamine solution, and the other was a solution of sterile water, which served as a placebo, with substance and hand order counterbalanced across participants. Participants were not informed of the placebo until the experiment had concluded, and were advised at the time that both were histamine prick tests. Once the experiment was completed participants were fully debriefed (see Fig. 2 for overview of timeline).
Itch induction
Itch was induced via a histamine skin prick test. One drop of 1% histamine dihydrochloride in aqueous solution was applied to the forearm and then the skin was superficially punctured using a lancet (Allergy Therapeutics, Worthing, UK). Saline solution was used for the placebo condition. Starting 2 min after the skin prick, participants rated the intensity of the itch sensation using the 0–100 general labelled magnitude scale (15, 16). This rating scale has verbal anchors with quasi-logarithmically placed labels of “no sensation” at 0, “barely detectable” at 1, “weak” at 6, “moderate” at 17, “strong” at 35, “very strong” at 53, and “strongest imaginable sensation” at 100. Participants gave 1 rating every 20 s until 10 min since skin prick onset had elapsed.
Statistical analysis
Before the statistical analysis using the R package afex (17), trials with very short (RTs < 150 ms, 1.9% of data) and very long RTs (> 1,000 ms, < 0.1% of data) were removed. RTs outside ± 2 SDs of a participant’s mean (3.4% of data) as well as incorrect responses (0.9% of data) were also excluded. Planned contrasts between threat and neutral trials in both validity types for each group were carried out using the R package emmeans (18). Reliability of the reaction time measure was estimated using the R package splithalfr (19). Using a Monte Carlo simulation approach, this involved splitting the data to produce 1,000 split half replications, which were then used to estimate Spearman-Brown coefficients as a measure of reliability. For all data and analysis scripts, see https://osf.io/u3fbz/.
RESULTS
As a manipulation check, we first analysed the itch ratings collected after the first skin prick. As expected, mean itch ratings were significantly higher (t(33.8)=4.1, p = 0.0003) following a histamine (14.8 ± 15.8) compared with the placebo skin prick (2.9 ± 3.8). When only considering the last itch rating of the time course (i.e. immediately before the spatial cueing task was started), this difference was also significant (histamine: 7.7 ± 12.7; placebo: 2.7 ± 4.9; t(39.1), p = 0.045). A similar pattern was observed for the second prick test, where mean itch ratings were significantly higher for histamine (17.2 ± 13.2) than for placebo (2.3 ± 2.9), t(29.4)=5.9, p = 2.262e–06.
Reliability of the spatial cueing task was high, with a mean Spearman-Brown coefficient of 0.96 (95% CI [0.93, 0.98]) for valid threat, 0.93 (95% CI [0.90, 0.96]) for invalid threat, 0.95 (95% CI [0.93, 0.97]) for valid neutral and 0.91 (95% CI [0.88, 0.94]) for invalid neutral trials. RTs were analysed using a mixed 2×2×2 analysis of variance (ANOVA), with Group (placebo, histamine) as the between-subjects factor, and Validity (valid, invalid) and Cue type (threat, control) as within subject factors. The ANOVA yielded a significant main effect of Validity (F(1,58)=79.82, p < 0.001) with invalid trials showing longer RTs (M=334, 95% CI [321, 346]) than valid trials (M=304, 95% CI [294, 314]) across both groups. Critically, a significant 3-way interaction of Validity×Cue type×Group (F(1,58)=6.09, p = 0.017) was also found. All other main effects and interactions of the ANOVA were not significant (all Fs<2.03, all p > 0.16). As can be seen in Table I and Fig. 3, the 3-way interaction was driven by the fact that in the histamine group there was a difference in the size of the validity effects for threat cues compared with neutral cues, whereas the size of the validity effect was not modulated by cue type in the placebo group.


Planned contrasts were then conducted to reveal whether AB occurs either at an early phase of attentional engagement or a later stage of attentional disengagement (see methods). These contrasts showed a significant effect of cue type on invalid trials within the histamine group only (p = 0.013), with shorter RTs for threat trials (336 ± 55) than for neutral trials (343 ± 52). None of the 3 other planned contrasts were significant; histamine_valid (p = 0.259); placebo_valid (p = 0.782); placebo_invalid (p = 0.503). Overall, the pattern of results suggests that attentional avoidance occurred in the later processing stages, with physical itch inducing avoidance of visual itch threats via facilitated disengagement of attention.
Finally, 2 post hoc exploratory analyses indicated that the magnitude of the AB score is not correlated with itch intensity (all p > 0.179) and that the side of itch stimulation (either left or right hand prick) did not interact with the other experimental factors (all p > 0.055, see Appendix S1 for more detail).
DISCUSSION
This study aimed to identify whether acute itch induced AB towards or away from threatening itch-related information in healthy individuals, and if so, whether it occurred in the early or later stages of processing. The main findings were that the experience of acute itch induces an AB away from, or avoidance of, visual itch threats and that this attentional avoidance occurs at a later processing stage in the form of facilitated disengagement of attention. At the same time, histamine may also have slowed down the disengagement of attention from the neutral words.
A robust main effect of validity was observed, in the form of longer RTs for targets that appeared in an un-cued location compared with a cued location. This is generally interpreted as reflecting additional costs arising from having to reorient attention away from the cued location towards the un-cued location (10). Critically, the size of these reorientation costs varied as a function of cue type (itch vs neutral) and group status (histamine vs placebo), with no differences observed in the placebo group, but smaller validity effects for threats compared with neutral cues in the histamine group.
The absence of a difference in the size of the validity effects in the placebo group indicates that threat and neutral words drew similar levels of attention when no physical itch was present. This is in line with an earlier report suggesting that visually presented itch-related stimuli are not processed in an attentionally biased manner in itch-free individuals (20). In the current study, the itch-related words may not have been perceived as threats by the participants because decontextualized words activate an array of possible meanings (21, 22). For example, without context, the word “skin” may activate the concept of itch, but also other skin-related aspects and sensations, such as skin smoothness, colour, temperature and pain.
In contrast, in the histamine group, the simultaneous experience of acute itch provides a context that shapes and constrains the interpretation of the threat words. Since the threat words were selected to be related to the experience of histamine-induce itch and participants encountered these words while they were still itching, is highly likely that the words specifically activated the intended itch-related concept, rather than a whole array of possible meanings (21, 23). This increases the likelihood that the words are interpreted as threats which are then attentionally avoided. At the same time, participants in the histamine group may realise that some words (i.e. the neutral words) in the study are not itch-related and thus do not remind them about the ongoing unpleasant itch sensation. Participants may come to see these neutral words as “safe cues”, which they attend to for longer, resulting in the hypervigilance pattern observed for neutral words in the histamine group. Overall, this pattern suggests that acute itch induces an attentional avoidance of visual itch threats. In addition to faster avoidant processing of the itch-related information, histamine may also have resulted in slowing down the attentional disengagement from the neutral information; possibly because this information could not be placed within the itch-context.
One advantage of the spatial cueing task is that planned contrasts can be used to clarify whether an AB occurs in early or later processing stages. The current data suggest that the pattern of attentional avoidance occurs in the later processing stages, in the form of faster disengagement from threat cues. Thus, acute itch seems to affect visual attention in the later, more strategic stage of information processing (24, 25). Furthermore, the pattern of results from the exploratory analyses (see Appendix S1) suggests that neither the severity nor the location of acute itch influences the pattern of reaction times on the spatial cueing task. This is similar to findings from the related domain of pain, where pain intensity tends to be unrelated to the magnitude of AB towards pain-related visual stimuli (3).
While the current study has provided evidence for avoidance in the later disengagement phase, this does not preclude the possibility of additional earlier effects. For example, the Vigilance-Avoidance hypothesis (26) posits that in anxious individuals, AB is initially directed towards threatening information, but that this is then followed by later avoidance. In support of this, a study on AB in anxiety (27) demonstrated using the spatial cueing task that highly anxious participants showed hypervigilance for threat-related images at a 100 ms stimulus onset asynchrony (SOA) in both the earlier (facilitated engagement) and later (impaired disengagement) processing stages. Highly anxious participants showed avoidance of threat-related images, again in both the earlier (slower attentional engagement) and later (faster attending away from threat) stages of processing when the SOA was increased to longer presentations of 200 and 500 ms. A similar process might occur when experiencing itch, in that avoidance was indicated in the current study when using a somewhat longer SOA (250 ms in the current study). Using a shorter SOA instead could well provide evidence of early hypervigilance.
Recent research from the related domain of pain suggests that, rather than being fixed and maladaptive, attentional biases towards pain are in fact better characterized as functional phenomena that flexibly adapt depending on context and motivation (23, 28). For example, according to the Threat Interpretation Model of Pain (TIMP, see (23)), a stimulus is only processed in an attentionally biased manner if is categorized as pain relevant and potentially threatening. In contrast, stimuli that are not perceived as pain-relevant, or as pain-relevant but not threatening, are assumed to be subject to normal attentional processing. Such a theoretical approach could explain the pattern observed in the present and previous AB studies on itch processing. In itch-free individuals, visual itch-related information is not processed in an attentionally biased manner (see placebo group of the current study, as well as (20)), because without the context of physical itch, the visual stimuli are either not interpreted as directly itch-related (e.g. due to semantic ambiguity) or because of a lack of perceived threat. In contrast, in participants already experiencing a physical itch, visual itch-related information is more likely to be perceived as threat and is therefore processed in an attentionally biased manner, as indicated by the pattern in the histamine group in the current study.
Interestingly, the TIMP predicts that the strength of the perceived threat determines the direction of AB, with moderate threats eliciting hypervigilance (in the form of delayed disengagement) and strong threats leading to avoidance (i.e. facilitated disengagement). This could explain why an earlier study (4) observed a hypervigilant pattern, whereas the current study observed avoidant attentional processing of visual itch threats. In the study by van Laarhoven et al. (4), the AB tasks (dot-probe and Stroop task) were started after most of the physical itch had already decayed in participants, resulting in only a moderate threat evaluation of visual itch stimuli and subsequent hypervigilant processing. In contrast, in the current study the physical itch sensation was more prevalent when the reaction time task was started, arguably leading to visual itch information being perceived as strong threats and therefore triggering attentional avoidance. Finally, the current study used threat words that specifically relate to the sensory experience of histamine-induced itch, whereas van Laarhoven et al. used stimuli that related to itch experiences encountered in daily life. This difference in focality of the employed visual stimuli might also influence the direction of an AB.
A largely unexplored question is how attentionally biased processing of visual itch threats interacts with chronic itch. There is some evidence that patients with psoriasis, where itch is major symptom, show increased attention to disease-related words (29). Vigilant-avoidant processing of pain-related information has been discussed as a vulnerability factor for development of chronic pain (30). Together with the avoidant processing pattern observed in the current study, this raises the possibility that attentionally biased processing might play a role in the chronification of itch as well. More AB studies involving patients are required to further explore this clinically relevant question. Another important aspect for future research is to test the extent to which such attentional avoidance as demonstrated in the current study in the laboratory extends to the natural environment, where attentional avoidance of itch threats may increase the risk of accidental exposure to pruritogens.
One limitation of the current study was that potential patterns of hypervigilance towards itch-related stimuli may have been missed because we used only one SOA of 250 ms. Future studies could systematically vary the length for which a threat cue is presented to investigate whether, for example, shorter SOAs lead to hypervigilant processing of itch-related stimuli. It could also be interesting to measure levels of anxiety, since this variable is known to affect AB (27). Another limitation was that participants felt only relatively weak levels of itch by the time the AB task was started.
In conclusion, the current study provided clear evidence that, in healthy volunteers, acute itch induces attentional avoidance of visual itch threats, in the form of facilitated attentional disengagement of itch information and/or delayed attentional disengagement of neutral information. Future research in this area should focus on the manipulation of different SOAs, as well as the presence of AB among populations with chronic itch.
ACKNOWLEDGEMENTS
We would like to thank the Psoriasis Association for their funding support (award number ST2/18). The funder had no influence over the design of our study. We would also like to thank the study participants.
This work was supported by a grant from the Psoriasis Association (award number ST2/18).
The authors have no conflicts of interest to declare.
REFERENCES
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci 2006; 7: 535–547.
- van Laarhoven AI, Kraaimaat FW, Wilder-Smith OH, Evers AW. Role of attentional focus on bodily sensations in sensitivity to itch and pain. Acta Derm Venereol 2010; 90: 46–51.
- Crombez G, Van Ryckeghem DML, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. Pain 2013; 154: 497–510.
- van Laarhoven AIM, van Damme S, Lavrijsen APM, van Ryckeghem DM, Crombez G, Evers AWM. Attentional processing of itch. Psychol Res 2018; 82: 876–888.
- van Laarhoven AIM, van Damme S, Lavrijsen ASPM, van Ryckeghem DM, Crombez G, Evers AWM. Do tonic itch and pain stimuli draw attention towards their location? Biomed Res Int 2017; 2017: 2031627–2031627.
- Posner MI. Orienting of attention. Q J Exp Psychol 1980; 32: 3–25.
- Grubert A, Righi LL, Eimer M. A unitary focus of spatial attention during attentional capture: Evidence from event-related brain potentials. J Vis 2013; 13: 1–11.
- Zhou Y, Liang L, Pan Y, Qian N, Zhang M. Sites of overt and covert attention define simultaneous spatial reference centers for visuomotor response. Scientific Reports 2017; 7: 46556.
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci 1990; 13: 25–42.
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull 2007; 133: 1–24.
- Cohen J. A power primer. Psychol Bull 1992; 112: 155–159.
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J Exp Psychol Gen 2001; 130: 681–700.
- Kosteletzky F, Namer B, Forster C, Handwerker HO. Impact of scratching on itch and sympathetic reflexes induced by cowhage (Mucuna pruriens) and histamine. Acta Derm Venereol 2009; 89: 271–277.
- van Heuven WJ, Mandera P, Keuleers E, Brysbaert M. SUBTLEX-UK: a new and improved word frequency database for British English. Q J Exp Psychol (Hove) 2014; 67: 1176–1190.
- Jones O, Schindler IC, Holle H. Assessing acute itch intensity: general labelled magnitude scale is more reliable than classic visual analogue scale. Acta Derm Venereol 2017; 97: 375–376.
- LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol 2009; 101: 1430–1443.
- Singmann H, Bolker B, Westfall J, Aust F, Ben-Shachar MS. afex: analysis of factorial experiments. R package version 1.0-1. 2021.
- Lenth RV. emmeans: estimated marginal means, aka least-squares means. R package version 1.7.0. 2021.
- Pronk T, Molenaar D, Wiers RW, Murre J. Methods to split cognitive task data for estimating split-half reliability: a comprehensive review and systematic assessment. Psychon Bull Rev 2021.
- Becker JM, Vreijling SR, Dobbinga S, Giesbers JJJ, Evers AWM, Veldhuijzen DS, et al. Attentional bias towards visual itch and pain stimuli in itch- and pain-free individuals? Acta Derm Venereol 2020; 100: adv00199.
- Holle H, Gunter TC. The role of iconic gestures in speech disambiguation: ERP evidence. J Cogn Neurosci 2007; 19: 1175–1192.
- Twilley LC, Dixon P. Meaning resolution processes for words: a parallel independent model. Psychon Bull Rev 2000; 7: 49–82.
- Todd J, Sharpe L, Johnson A, Nicholson Perry K, Colagiuri B, Dear BF. Towards a new model of attentional biases in the development, maintenance, and management of pain. Pain 2015; 156: 1589–1600.
- Shiffrin RM, Schneider W. Controlled and automatic human information-processing. 2. perceptual learning, automatic attending, and a general theory. Psychol Rev 1977; 84: 127–190.
- Schneider W, Chein JM. Controlled & automatic processing: behavior, theory, and biological mechanisms. Cogn Sci 2003; 27: 525–559.
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther 1998; 36: 809–848.
- Koster EH, Crombez G, Verschuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: facilitated engagement, impaired disengagement, and attentional avoidance. Behav Res Ther 2006; 44: 1757–1771.
- Van Ryckeghem DML, Noel M, Sharpe L, Pincus T, Van Damme S. Cognitive biases in pain: an integrated functional-contextual framework. Pain 2019; 160: 1489–1493.
- Fortune DG, Richards HL, Corrin A, Taylor RJ, Griffiths CE, Main CJ. Attentional bias for psoriasis-specific and psychosocial threat in patients with psoriasis. J Behav Med 2003; 26: 211–224.
- Broadbent P, Schoth DE, Liossi C. Association between attentional bias to experimentally induced pain and to pain-related words in healthy individuals: the moderating role of interpretation bias. Pain 2022; 163: 319–333.