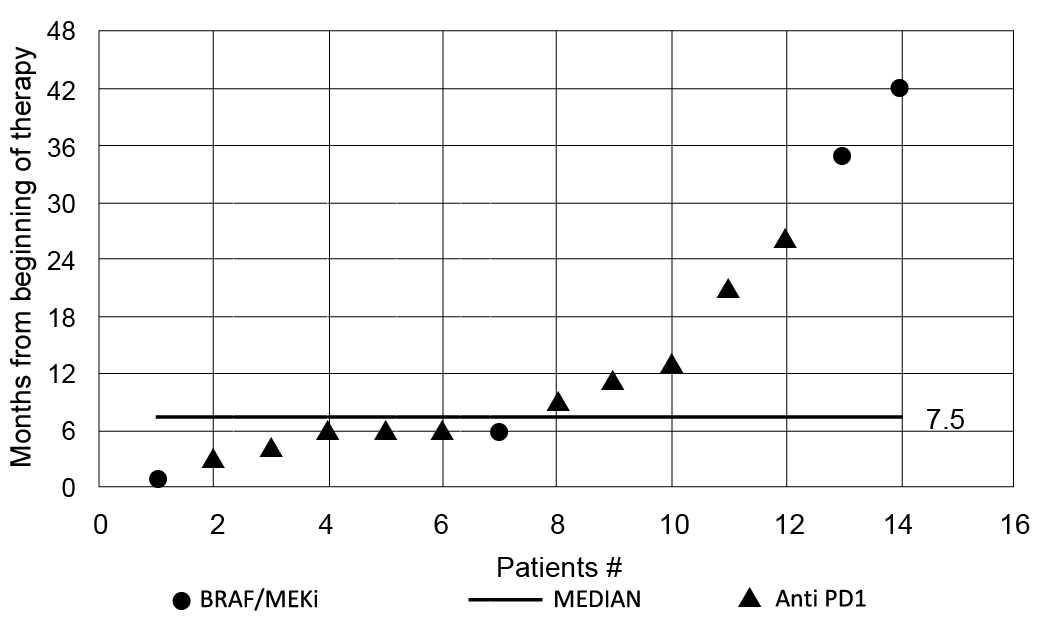
Fig. 1. Distribution curve of the time of onset of drug-induced vitiligo from the start of therapies, ranging from 1 to 42 months, with a median time of appearance of cutaneous lesions of 7.5 months from the start of treatments.
1Dermatologic Clinic, 2Section of Surgical Pathology, Department of Medical Sciences, 3Department of Dermatological Surgery, University of Turin, Turin, and 4Medical Oncology Unit, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari , Italy
#Both authors contributed equally.
Current therapies for metastatic melanoma (anti-PD1 and BRAF/MEK inhibitors) can cause drug-induced vitiligo. The aim of this study is to dermatologically define and histologically characterize this new type of vitiligo, and assess the clinical course of the disease. Fourteen patients with metastatic melanoma treated with immune or targeted therapy were included in a dataset evaluating clinical data, vitiligo description and histopathological features. Vitiligo-like lesions occurred after a mean of 7.5 months from the start of the therapies (range 1–42 months), with a prevalence of the non-segmental variant (71.4%). Fifty percent of patients showed a clinical response (4 complete response and 3 partial response), 35.7% had stable disease, and one patient died after disease progression. Median survival from the start of the therapies was 32.5 months. Drug-induced vitiligo can be related to both immune and targeted therapies, is associated with a favourable prognosis, and has clinical characteristics different from the classical form.
Key words: vitiligo; targeted therapy; immunotherapy; melanoma; survival.
Accepted Sep 18, 2019; E-published Sep 18, 2019
Acta Derm Venereol 2020; 100: XX–XX.
Corr: Simone Ribero, Dermatologic Clinic, University of Turin, Via Cherasco 23, IT-10126 Turin, Italy. E-mail: simone.ribero@unito.it
There is a new type of vitiligo called “drug-induced” because it appears in some patients treated for metastatic melanoma with immune and targeted therapy. This study analysed this phenomenon from a clinical, prognostic and histopathological aspects. This is the first study to classify drug-induced vitiligo according to the European Guidelines for the management of vitiligo, revealing that the most frequent subtype is the non-segmental form (71.4%). Moreover, the study showed that vitiligo can also develop in patients treated with targeted therapies, and the patients who develop vitiligo had a favorable prognosis, with a median overall survival of 57 months.
Vulgar vitiligo is a primary dermatological condition, with a prevalence in the general population of approximately 0.5–1% (1). Vitiligo-like patches have been reported in patients with melanoma as a probable sign of immune activation towards cancer cells. Its prevalence in all stages of melanoma is low (between 2.8% and 4.1% of cases) (2, 3). It is defined as a cutaneous hypopigmentation due to the loss of functional melanocytes, since this has been hypothesized as being associated with a better prognosis (2). Induction of vitiligo is considered to be strictly related to the development of an anti-tumour immune response, as some of the antigens expressed by tumour cells (MART-1, gp100, tyrosinase 1-2) are also expressed by healthy melanocytes (4, 5). The appearance of vitiligo has been reported during immunotherapy for melanoma, but never under targeted therapies.
Anti-PD1 immunotherapy and targeted therapy with BRAF and MEK inhibitors have markedly improved the prognosis of the advanced disease phase in patients with melanoma. However, these drugs can induce the development of adverse effects, many of which involve the skin (maculo-papular eruption, pruritus, hypopigmentation, xerosis, photo-sensitivity, keratosis and vitiligo) (6).
The pathogenesis of these side-effects appears to be related to the mechanism of action of these drugs (immune-stimulation for anti-PD1 antibodies and inhibition of the MAPKinase tumour-related activation for the combo targeted regimens), owing to the fact that some of the signalling pathways targeted by the therapies are also involved in the regulation of cutaneous homeostasis (7).
The first immune therapeutic drug used for metastatic melanoma (IL-2) had already shown a higher prevalence of vitiligo (43%) compared with non-drug-induced vitiligo (8).
Despite these differences, few studies have analysed and characterized this phenomenon from the dermatological point of view focusing on drug-induced vitiligo.
This study describes a series of patients with metastatic melanoma who developed vitiligo-like lesions. The aim of the study was to describe better, from a dermatological point of view, the clinical characteristics of vitiligo (morphology and topography, pattern of clinical lesions, time of onset with respect to the start of the therapies) classified in the different forms according to European Guidelines for the management of vitiligo (9). Additional secondary objectives were to describe the different forms of drug-induced vitiligo surrounding melanoma metastases or in previously healthy skin.
The third aim of this study was to correlate the appearance of vitiligo with the clinical course, taking into account both overall response and survival.
A retrospective cohort study was performed at the Dermatological Clinic of the University of Turin. All patients treated for metastatic melanoma who had developed vitiligo-like lesions after the diagnosis of advanced stage III–stage IV were extracted from the clinical melanoma database. Between 2016 and 2018, those patients who developed vitiligo during targeted or immunotherapy from an unresectable stage III or a stage IV melanoma were identified.
Careful clinical observation (with Wood’s lamp devices) was performed at every hospital visit or during infusional therapy or drug re-supply. Patients’ demographic, clinical, pathological and follow-up information were prospectively recorded and updated on an internal database.
The clinical classification was performed by referring to the European Guidelines for the management of vitiligo, differentiating among non-segmental forms (focal, mucosal, acrofacial, generalized, universal), segmental forms (focal, mucosal, uni-, bi- or pluri-segmental) and mixed forms (segmental and non-segmental) (9).
The response to treatment was assessed based on Response Evaluation Criteria In Solid Tumours 1.1 (RECIST) measures through radiological examinations (computed tomography (CT), positron-emission tomography (PET)) performed every 4 months.
Punch biopsies were performed from the hypopigmented patches developed in healthy skin. At the same time, excisional biopsies of the regressed metastases surrounded by vitiligo phenomenon were analysed. For each group, the histopathological features were assessed and the inflammatory infiltrate compared.
Of the total number of patients treated for advanced stage III–stage IV melanoma in our centre from 2016 to 2018 (n = 314; 150 first-line targeted therapy and 164 immunotherapy), 14 were diagnosed with drug-induced vitiligo. Of these, 10 (71.4%) had been treated previously with anti-PD1 and 4 with a combination of BRAF and MEK inhibition (28.5%).
Ten were males (71.4%) and 4 females (28.5%). In total, vitiligo appeared after a median of 7.5 months from the start of the therapies (range 1–42 months) (Fig. 1). All the cases of drug-induced vitiligo that appeared before 6 months (n = 3) were non-segmental (2 generalized and 1 acrofacial). Concerning the staging, 2 patients showed stage IIIc, who received anti-PD1 for the presence of disease that was not surgically treatable (1 with “in transit” metastases, the other with skin plus regional nodal metastases). The other 12 patients had stage IV (1 patient stage m1a, 3 patients stage m1b, 6 patients stage m1c, and 2 patients stage m1d.)

Fig. 1. Distribution curve of the time of onset of drug-induced vitiligo from the start of therapies, ranging from 1 to 42 months, with a median time of appearance of cutaneous lesions of 7.5 months from the start of treatments.
Concerning dermatological clinical characteristics, most of the cases of drug-induced vitiligo were represented by the non-segmental variant (10/14, 71.4%), segmental (3/14, 21.4%) and mixed (2/14, 14.3%) (Fig. 2). Among the non-segmental variants, 4 patients showed the generalized form, 4 acrofacial and 2 focal (Table I).
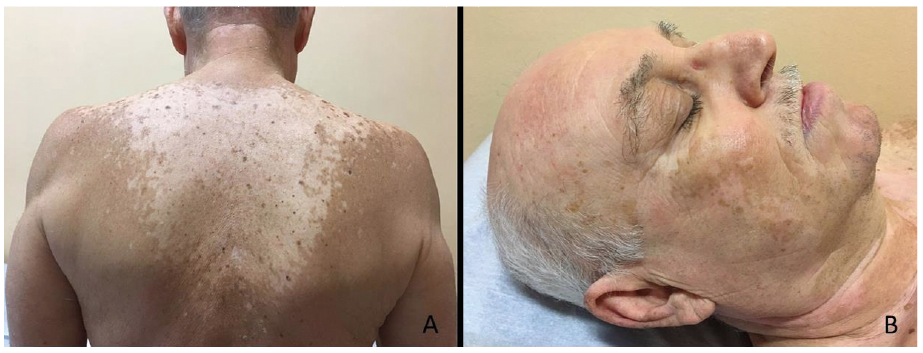
Fig. 2. Patient number 14. (A) Non-segmental generalized vitiligo of the upper trunk/dorsum and (B) head /neck, characterized by a typical symmetrical distribution of confluent macules in patches with jagged and irregular edges. The patient was treated with immunotherapy, obtaining a complete response, with an overall survival of 21 months. Permission is given to publish these photos.
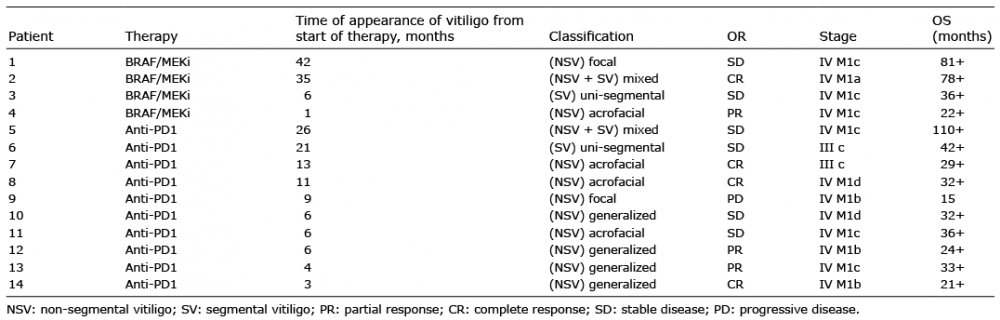
Table I. Time of onset and classification of drug-induced vitiligo developed by patients with metastatic melanoma treated with anti-PD1 drugs and targeted therapy (BRAF/MEKi) according to the European Guidelines for the management of vitiligo, specifying overall responses (OR) and overall survival (OS)
The lesions appeared as speckled hypochromic macules confluent into patchy stains with shaded and jagged edges. Photo-exposed areas were the most involved (face, neck, hands and arms).
Concerning the prevalence of sub-classification and the drug involved, BRAF and MEK inhibitors were associated with 2 non-segmental (1 focal and 1 acrofacial), 1 segmental and 1 mixed. Anti-PD1 patients developed 1 segmental, 1 mixed and 8 non-segmental vitiligo (4 generalized, 3 acrofacial and 1 focal).
Clinical course
A drug response was observed for 7/14 patients, 4 of whom had a complete response (3 with anti-PD1 and 1 with BRAF and MEK inhibitors) and 3 a partial response (2 anti-PD1 and 1 BRAF and MEK inhibitors). Furthermore, 5 patients had stable disease (4 anti-PD1 and 1 BRAF and MEK inhibitors). One patient developed progressive disease and died. After a median of 32.5 months (range 15–110 months) 13 patients were alive, 3 of whom had survived longer than 3 years. Patients on a combination of BRAF and MEK inhibition therapy who developed drug-induced vitiligo were still alive after a mean of 57 months (range 22–81 months) (Table I). The only patient who progressed and died during the follow-up had developed only a focal vitiligo (non-segmental); a 2-cm patch on the dorsum of the right hand. Concerning the 5 patients in stable disease, 3 had developed a late-onset drug-induced vitiligo (after 21 months from the start of therapy). For complete response, the median onset of drug-induced vitiligo was 12 months from the start of the therapy, with a wide range of time of onset (3–35 months).
Pathology
All of the areas of vitiligo were documented histologically. In particular, the specimens were classified as vitiligo arising on previous skin metastases, or vitiligo arising on previously normal skin. In both types of lesion, a complete loss of melanin pigment was observed in epidermal keratinocytes and an absence or a strong reduction in the number of melanocytes at the basal epidermal layer. Histology of those vitiligo lesions arising from previous skin metastases revealed a context of post-inflammatory dermal melanosis, suggesting regression of the melanocytic lesion, within a dense band-like infiltrate located in the upper dermis composed of melanophages mixed with lymphocytes and plasma cells (Fig. 3A, B). By contrast, drug-induced vitiligo appearing in normal skin, while treated with immunotherapy and targeted therapy, revealed the presence of basal layer vacuolization, mild perivascular inflammatory infiltrate, rare eosinophils and melanophages. The loss/reduction of melanocytes was also highlighted by immune-histochemical colouration with antibodies against MELAN-A (Fig. 3C, D).
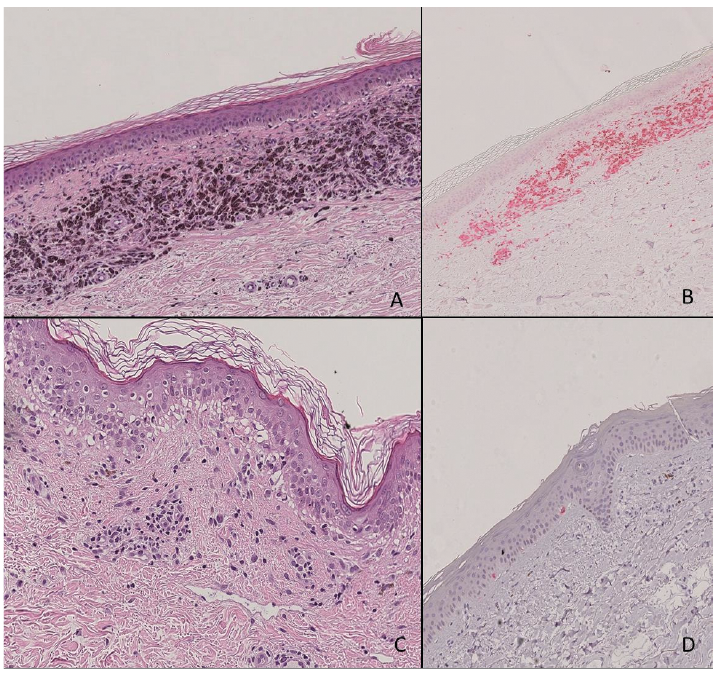
Fig. 3. (A) Drug-induced vitiligo in melanoma skin metastases. Dermal melanosis suggesting regression of melanocytic lesion, within a dense band-like infiltrate of cells in the upper dermis, consisting predominantly of melanophages mixed with lymphocytes and rare plasma cells. (B) Immuno-histochemical staining shows CD4-positive lymphocyte and melanophages in a band-like distribution. (C) Drug-induced vitiligo in non-involved skin of patient treated with immunotherapy: presence of basal layer vacuolization, mild superficial perivascular lymphocytic infiltrate with rare eosinophils and occasional melanophages. (D) Melan A- immuno-histochemical staining highlighted the almost complete disappearance of melanocytes in the basal layer of the epidermis.
The appearance of vitiligo-like lesions is a reported side-effect of immunotherapy, characteristically appearing in patients with metastatic melanoma; however, only a very few studies have analysed this condition from the dermatological point of view (7).
The current study describes 14 patients with vitiligo-like lesions who were treated with targeted therapy and immunotherapy. To our knowledge, this is the first study to classify drug-induced vitiligo according to the European Guidelines for the management of vitiligo, showing that the most frequent subtype is the non-segmental form (71.4%), particularly the generalized (40%) and acro-facial (40%) forms (9).
Regarding vulgar vitiligo, the most frequent form is the non-segmental one, whereas lesions usually show-ed a bilateral distribution in an acrofacial pattern, or scattered symmetrically over the entire body, evolving over time. Non-segmental vitiligo can initially have an acrofacial distribution, but it usually tends to progress to a generalized form (10).
It has been reported that, in contrast to classical vitiligo, in which the genitalia, wrists and perioral region are frequently involved (probably due to a Koebner phenomenon), drug-induced vitiligo is localized in photo-exposed areas, such as the face, neck, hands and arms, thus excluding a traumatic pathogenesis. These results are in line with Larsabal et al. (11), and we described the morphology of the patches characterized by speckled hypopigmented macules confluent into patchy stains with shaded and jagged edges, in contrast to the more homogeneous hypochromic patches with sharp edges, typical of vulgar vitiligo.
When comparing drug-induced vitiligo with melanoma-induced vitiligo (not associated with drugs), we found that the distribution of the latter was non-segmental with a prevalence of the focal form (78%) (2).
Morphologically, those lesions were similar to the drug-induced vitiligo described here, with hypochromic patches not well-demarcated with irregularly shaped borders and a pale colour. Treatment of metastatic melanoma has changed in the last few years (12). Despite the well-known appearance of vitiligo under immunotherapy, we describe here, for the first time, that vitiligo can also develop in patients treated with targeted therapies (4 out of 14 in our series). This phenomenon is less frequent than in immunotherapy due to the immune-involving drug mechanism. Targeted therapy-induced vitiligo can be explained by the effect on immune system cells related to the blockage of cancer cells by BRAF and MEK. In fact, targeted therapies have been shown to induce modifications in the expression of some surface molecules, thus affecting the melanoma-specific immune response at the level of antigen-specific T-lymphocyte response (13).
Targeted therapies increase the expression of MHC class I and II, of melanocyte differentiation antigens (MDA) and of PD-L1, and reduce CD200 mRNA levels in melanoma cells, preventing the inhibitory effect on dendritic cells (14). Furthermore, BRAF and MEK inhibitors have some effects on tumour microenvironment, reducing the levels of VEGF and Il-6/10 (15) and on tumour infiltrating lymphocytes (TILs), in which the CD8+ component is consistently increased (16).
The second aim of the current study was to identify pathological features related to the appearance of different drug-induced vitiligo lesions. Two separate patterns were identified, one in the presence of vitiligo lesions in proximity of previous metastases characterized by inflammatory infiltration and melanophages in a band-like disposition. The second pattern described the vitiligo lesion appearing on healthy skin during treatment. In these cases, the inflammatory infiltrate was mild or low and had a perivascular disposition.
Finally, we confirmed literature review on the favour-able prognosis of drug-induced vitiligo (7, 17, 18). In our patient series, all 4 patients treated with a combination of BRAF and MEK inhibitors were still alive after a median of 57 months (range 22–81 months), and, with regard to patients treated with anti-PD1, only one of the 10 patients died.
These figures underline the favourable prognosis related to drug-induced vitiligo (19). We therefore consider that vitiligo-like lesions represent an immune-mediated phenomenon, which develops over time in patients with a longer survival.
This paper describes, for the first time, the appearance of drug-induced vitiligo under BRAF and MEK inhibition therapy. The patients included in this study had a median survival of 50 months from the appearance of vitiligo.
Since drug-induced vitiligo can be considered a sign of systemic activation of the immune system, our survival data concerning patients under targeted therapy were in accordance with the evidence of an increase in tumour-infiltrating lymphocytes following BRAF/MEKi that may be related to more factors than the microenvironment of the cancer cells (15, 20).
Long-term responses associated with vitiligo have been reported in mouse models and human patients with melanoma (21). Despite the relatively low number of cases included, 13/14 were responding after a median of 18 months. In this respect, it was found that the immunological activation elicited by immunotherapy and targeted therapies took several months to manifest clinically, thus drug-induced vitiligo could be considered a belated adverse event (median 7.5 months in our cohort). However, the data are limited and a larger series of patients is needed to confirm this theory.
Another possible limitation of this study was that it was not possible to distinguish which patients under systemic treatment had developed a drug-induced vitiligo from those who would have developed vitiligo without the treatment. In fact, it has been reported that a proportion (up to 4%) of patients with metastatic melanoma developed vitiligo during the clinical course even in the pre-systemic era (2).
In conclusion, drug-induced vitiligo is a clear entity with a specific morphology and topography, which should be differentiated from melanoma-induced vitiligo (not associated with drugs) and from vulgar vitiligo. Further research is needed to determine the biological origin of this phenomenon in order to evaluate its prognostic value.
The authors thank Professor Roy Howse for language revision.