Table I. Profile of 329 patients with atopic dermatitis
1Department of Dermatology, 2Division of Skin Surface Sensing, Graduate School of Medical Sciences, Kyushu University, 3Department of Dermatology, National Hospital Organization Fukuoka-higashi Medical Center, Fukuoka, Japan, and 4Department of Dermatology, Health Sciences University, Ulaanbaatar, Mongolia
The Patient-Oriented Eczema Measure (POEM, 0–28 points) is a self-assessed, repeatable measurement tool for measuring atopic dermatitis (AD) severity. How-ever, whether POEM score is influenced by allergic comorbidities and whether POEM’s severity banding is applicable in web-based surveys for AD remain unclear. A web-based questionnaire survey was conducted in 329 patients with AD. POEM, self-reported severity of AD, and comorbidity of allergic diseases including asthma, pollen rhinitis, allergic conjunctivitis, and food allergy were assessed. POEM scores were not affected by a history of comorbid allergic diseases. The severity banding for POEM scores on the web-based survey was as follows: clear/almost clear = 0, mild = 1–8, moderate = 9–21, and severe/very severe = 22–28, which was comparable to previous banding. These results suggest that POEM is useful for determining AD severity, even in web-based surveys. Patients with POEM scores above 9 points may be grouped into moderate, severe, and very severe AD.
Key words: allergic comorbidity, atopic dermatitis, Patient-Oriented Eczema Measure, self-assessed measurement, web-based survey.
Accepted May 19, 2020; Epub ahead of print May 25, 2020
Acta Derm Venereol 2020; 100: adv00159.
Corr: Makiko Kido-Nakahara, Department of Dermatology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan. E-mail: macky@dermatol.med.kyushu-u.ac.jp
The Patient-Oriented Eczema Measure (POEM) is a self-assessed, repeatable measurement tool for measuring atopic dermatitis severity. A web-based questionnaire survey was conducted with POEM in 329 patients with atopic dermatitis. POEM scores were not affected by a history of comorbid allergic diseases including asthma, pollen rhinitis, allergic conjunctivitis, and food allergy. POEM was shown to be a specific measurement tool for atopic dermatitis irrespective of allergic comorbidities. POEM is useful for determining atopic dermatitis severity, not only in clinical settings, but also in a web-based survey. Patients with POEM scores > 9 points may be grouped into moderate to very severe atopic dermatitis.
Atopic dermatitis (AD) is a common, chronic, severely pruritic, eczematous skin disease that markedly reduces the quality of life of affected patients (1, 2). It is characterized by intense itch, skin inflammation, and skin barrier dysfunction (xerosis) (1, 3). Members of the Harmonizing Outcome Measures for ECZEMA (HOME) group reached a consensus that outcome domains for AD trials should include clinical signs, symptoms, long-term control of flares, and quality of life (4), and it has been recommended that clinical signs should be measured using the Eczema Area and Severity Index (5). To measure the core domain of symptoms in AD, the Patient-Oriented Eczema Measure (POEM) was recommended (6, 7).
POEM is a self-assessed, repeatable, and well-validated tool for measuring AD severity (8–10). It is composed of 7 questions evaluating itch, skin dryness, and sleep disturbance (6, 7, 10). It was specifically developed to measure self-assessed AD activity, but the influence on POEM scores of allergic comorbidities has not yet been evaluated. Therefore, one of our aims in this study was to clarify whether allergic diseases, such as asthma, pollen rhinitis, allergic conjunctivitis, and food allergy, could potentially influence the POEM score.
Charman et al. (10) also reported the proposed optimal banding for POEM score in AD patients in the UK as follows: 0–2 (clear/almost clear), 3–7 (mild), 8–16 (moderate), 17–24 (severe), and 25–28 (very severe). A similar optimal banding range was obtained in Japanese patients with AD: 0–2 (clear/almost clear), 3–8 (mild), 9–18 (moderate), and 19–28 (severe/very severe) (11). In these studies, paper- and pencil-administered questionnaires were used to collect information of POEM scores. Recent developments in information technology have enabled worldwide web-based surveys, which provide an attractive alternative due to their range of advantages (12). However, it has also been reported that important technical and methodological issues should be carefully considered before widespread implementation of such surveys (13–15). Regarding POEM, it has not yet been validated whether the proposed POEM optimal bandings are applicable in web-based surveys in AD. To resolve these issues, a web-based questionnaire survey was conducted in patients with AD.
Survey methods and patients
This study was conducted from June 2017 to December 2017 using a self-answered anonymous online survey by AD patients who visited our educational websites for AD (http://www.kyudai-derm.org/atopy/index.html or http://www.kyudai-derm.org/kayumi/index.html, http://www.kyudai-derm.org/atopy_care/index.html) and agreed to complete a questionnaire. The study was approved by the ethics committee of Kyushu University (27-326).
Questionnaire
The questionnaire consisted of 5 sections written in Japanese. The first section included questions regarding a history of AD and comorbid allergic diseases: asthma, allergic rhinitis, allergic conjunctivitis, and food allergy. Only AD patients who had been diagnosed with AD by dermatologists were recruited to this study. History of comorbid allergic diseases was divided into 3 categories: having been diagnosed by a physician with symptoms within 1 year (2 points), having been diagnosed by a physician without symptoms within 1 year (1 point), and never having been diagnosed by a physician (0 points).
Sections 2–5 consisted of 4 validated measures for assessing disease severity: POEM, Global Question (GQ), itch numerical rating scale (NRS), and itch verbal rating scale (VRS). GQ was proposed by Charman et al. (10) and involves asking patients to evaluate their overall AD condition among 5 different severity grades: clear (0), mild (1), moderate (2), severe (3), and very severe (4). For itch-NRS, the patients assess the intensity of pruritus from 0 (no pruritus) to 10 (the worst itch imaginable) in the past 24 h. Itch-VRS has 5 points: no pruritus (0), mild pruritus (1), moderate pruritus (2), severe pruritus (3), and very severe pruritus (4).
Data processing and statistical analysis
All data were analysed statistically using Microsoft Excel 2016 (Microsoft Corporation, Tokyo, Japan) and GraphPad Prism (GraphPad Software, San Diego, CA, USA). Descriptive statistics for quantitative variables are presented as mean and standard error. Spearman’s rank correlation test was used to analyse the association of POEM and other parameters. One-way analysis of variance (ANOVA) with Bonferroni’s post hoc test was used to analyse the association of POEM and history of comorbidities. In these analyses, POEM score was used as a continuous variable. Determination of the 4 POEM categories was performed as previously reported (11). The mean, mode, and median GQ scores were used to group the POEM scores into possible severity strata, and the kappa coefficient of agreement was calculated for each set of bands. Statistical significance was set at p < 0.05.
A total of 331 patients who had been diagnosed with AD by a dermatologist visited our websites for AD and agreed to complete the questionnaire. Two subjects aged < 10 years were excluded from the analyses due to the limited reliability of their results given their age. Finally, 329 subjects were thus included in the analyses (99.4%). In total, 149 were male and 180 were female, with 311 adults aged ≥ 16 years and 18 children aged 10–15 years (mean age 33.0 years, range 10–69 years) (Table I). Overall, 325 out of the 329 individuals with AD (98.8%) had experienced AD symptoms within the past year. The profile and allergic comorbidities of the 329 patients with AD are shown in Table I.

Table I. Profile of 329 patients with atopic dermatitis
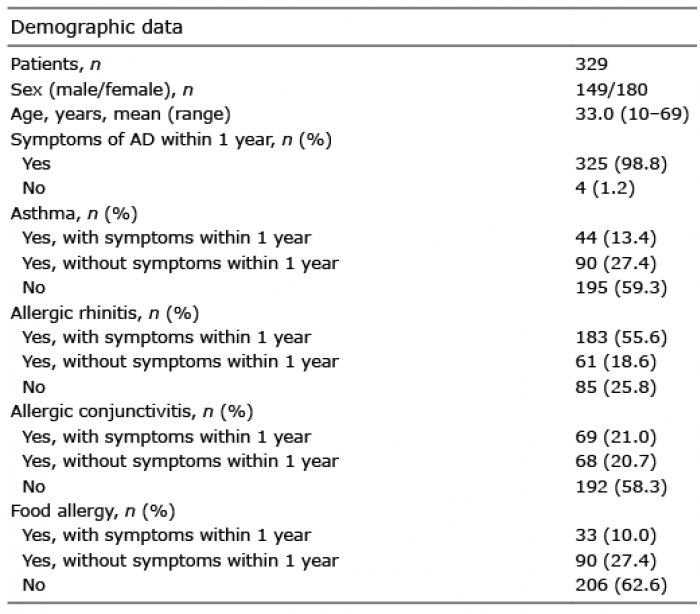
POEM scores significantly correlated with GQ scores (Spearman’s rank correlation test, r = 0.628, p < 0.001; Fig. 1A), itch-VRS scores (r = 0.551, p < 0.001; Fig. 1B), and itch-NRS scores (r = 0.581, p < 0.001; Fig. 1C), as reported previously (10, 11). POEM scores significantly increased in line with GQ severity (1-way ANOVA, p < 0,001; Fig. 1D).
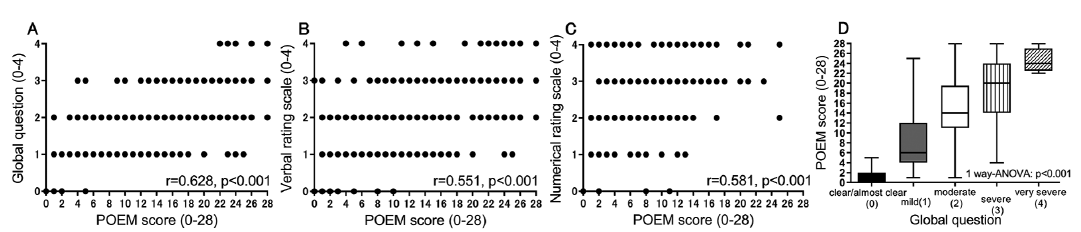
Fig. 1. Comparison between Patient-Oriented Eczema Measure (POEM) and Global Question (GQ) scores. (A) POEM and (B) itch visual analogue scale (VRS), and (C) POEM and itch numerical rating scale (NRS). (D) Relationship between the categories of POEM and GQ. ANOVA: analysis of variance.
The influence of other comorbid allergic diseases on POEM scores was then assessed by dividing subjects into 3 subgroups (patients with symptoms within the past year, patients without symptoms within the past year, and patients without a history of comorbid disease) in asthma (Fig. 2A), allergic rhinitis (Fig. 2B), allergic conjunctivitis (Fig. 2C), and food allergy (Fig. 2D). There were no significant differences in POEM scores in each comorbidity. Furthermore, multiple combinations of the 4 comorbid allergic diseases did not significantly influence POEM scores (Fig. 3).
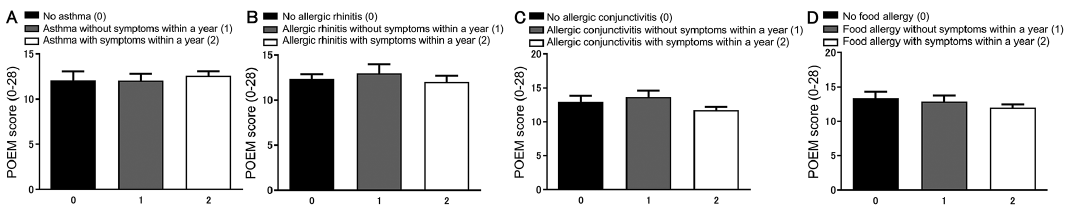
Fig. 2. Patient-Oriented Eczema Measure (POEM) of atopic dermatitis patients with/without (A) asthma, (B) allergic rhinitis, (C) allergic conjunctivitis, or (D) food allergy.
Fig. 3. Scores of Patient-Oriented Eczema Measure (POEM) based on the number of allergic comorbidities. It was examined whether the number of allergic comorbidities (asthma, allergic rhinitis, allergic conjunctivitis or food allergy) in patients with atopic dermatitis (AD) affects the POEM scores.
The mean, median, and mode of GQ score were calculated for each POEM score to estimate the cut-off values for the categorization of POEM scores into bands (Table II). The appropriate thresholds were suggested to be 1–2 for between clear and mild, 9–10 for between mild and moderate, and 22–23 for between moderate and severe/very severe. With these candidate thresholds, the optimal banding for POEM scores with the highest kappa coefficient agreement was chosen as the most appropriate optimal banding. The proposed optimal banding is as follows: clear/almost clear 0, mild 1–8, moderate 9–21, and severe/very severe 22–28 (Table III).
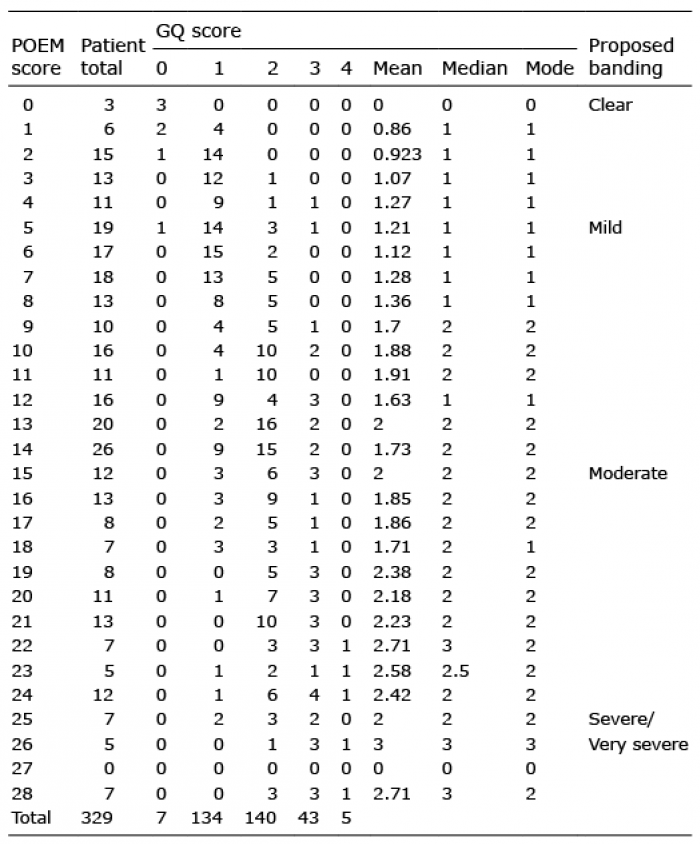
Table II. Number of patients with each Patient-Oriented Eczema Measure (POEM) score and corresponding global patient-rated disease severity scores (Global Question; GQ)
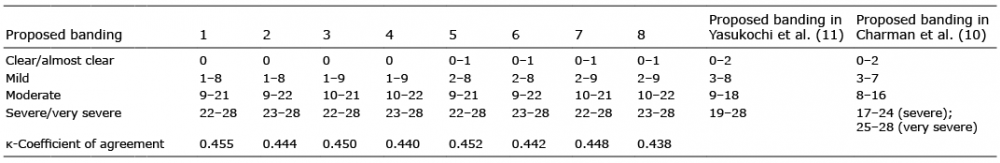
Table III. Kappa coefficient of agreement for different sets of Patient-Oriented Eczema Measure scores; the shaded area shows the final banding that was adopted. As references, the proposed banding of Yashukochi et al. (11) and Charman et al. (10) are also listed
This study performed a web-based questionnaire survey on patients with AD and evaluated POEM, GQ, itch-NRS, itch-VRS, and a history of other allergic comorbidities. Similar to previous hospital-based studies (10, 11), the results showed that POEM scores had significant correlations with GQ, itch-NRS, and itch-VRS, in the web-based questionnaire survey. In contrast, a recent or past history of allergic diseases did not significantly affect the POEM scores. Hon et al. (16) examined POEM and the Pediatric Allergic Disease Quality of Life Questionnaire (PADQLQ: quality of life score for children with allergic disease) for childhood AD and reported that POEM scores had almost no association with the severity of other allergic diseases. These results, including those of the current study, suggest that POEM is a rather specific measurement tool for AD, irrespective of allergic comorbidities, and that it is also applicable for measuring the severity of AD, even in web surveys.
The present web-based survey suggested that the optimal banding for POEM scores is as follows: clear/almost clear = 0, mild = 1–8, moderate = 9–21, and severe/very severe = 22–28. In a previous hospital-based study, the following optimal banding was proposed: clear/almost clear = 0–2, mild = 3–8, moderate = 9–18, and severe/very severe = 19–28 (11). These 2 optimal bandings of POEM scores are not completely consistent, but differ by no more than 3 points in each category. It has been reported that the minimal clinically important difference of POEM scores was 3.4 (9); therefore, the 3-point difference of the optimal bandings for POEM scores could be acceptable. Thus, it was considered that the previously reported bandings of POEM for use in a hospital setting could also be used in web-based surveys. It has been reported that important technical and methodological issues in web-based surveys should be considered with care (12–14). Further research is required to define the evaluation of POEM scores in web-based studies.
It would be important to discriminate the “moderate and severe/very severe” patients from the “clear and mild” ones with regard to selecting appropriate treatments. According to European, American, and Japanese guidelines for the treatment of AD (3, 17, 18), systemic therapy including cyclosporine and dupilumab should be considered for moderate to severe refractory patients. Furthermore, these patients are a particular target for newly developed drugs. In both the current web-based study and our previous study in a clinical setting, patients reporting POEM scores of more than 9 points should be considered to have more than moderate severity among Japanese subjects (Table III). In web-based studies targeting newly developed drugs, it would be useful and appropriate to extract “moderate and severe/very severe” patients using a POEM score of 9 points among Japanese patients.
In conclusion, POEM was shown to be a specific measurement tool for AD irrespective of allergic comorbidities, not only in clinical settings, but also in a web-based survey. Patients with POEM of more than 9 points could be regarded as having moderate or severe/very severe AD, at least in the Japanese population.
The authors thank Mr. Jun Kishi for his technical assistance.