Granulomatous Reaction to Cosmetic Soft Tissue Filler with Late-onset Inflammatory Response
1Department of Dermatology, Ruprecht-Karls-University of Heidelberg, Im Neuenheimer Feld 440, DE-69120 Heidelberg, 2Department of Radiology, University Hospital Heidelberg, and 3Department of Radiology, German Cancer Research Center, Heidelberg, Germany. *E-mail: anke.lonsdorf@med.uni-heidelberg.de
Accepted Jul 9, 2020; Epub ahead of print Jul 29, 2020
Acta Derm Venereol 2020; 100: adv00243.
Licensed dermal fillers are generally considered to be safe and well tolerated, but adverse side-effects may occasionally occur. The mean latency until the first adverse reaction is 14.6 ± 5.27 months (1). Common early complications include delayed hypersensitivity reactions, swelling (60.1%), redness and pain (22.6%) (2). Chronic granulomatous foreign body reaction and nodule formation (33.7%) are common late complications (2), while a late inflammatory response syndrome (LIRS), occurring months or even years after hyaluronic acid (HA) injection, is considered a rare adverse event (0.42%) (3, 4).
We report here a patient with a rare course of chronic granulomatous reaction and acute late-onset inflammation subsequent to a cosmetic contouring procedure with soft tissue filler 17 years earlier.
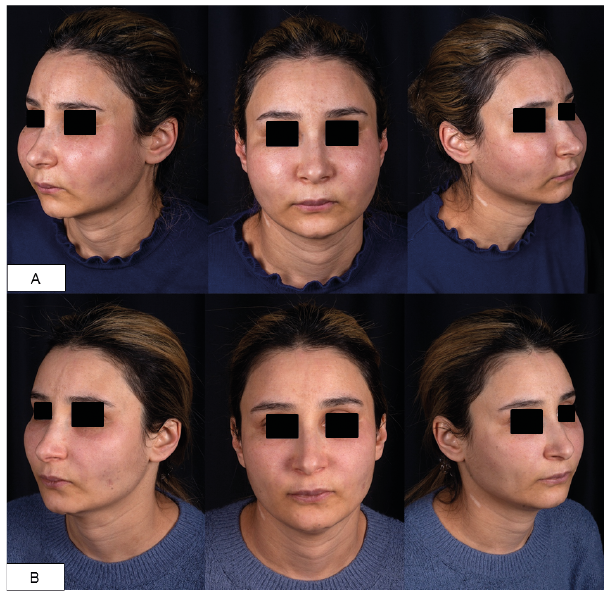
A 37-year-old woman presented to our clinic with a 2-week history of painful oedematous swelling and burning sensation of her face without spontaneous resolution. Seventeen years earlier, a firm permanent induration of both cheeks had reportedly occurred a few months after injection of dermal filler, allegedly polymethylmeth-acrylate (PMMA), into her cheeks and glabella by a dermatologist in Syria. Physical examination revealed a symmetrical firm, slightly overheated induration of the skin of both cheeks, accompanied by oedematous swelling and mild erythema (Fig. 1A). Local lymph-adenopathy, demarcated nodules or fluctuation were not palpable. Routine laboratory findings were all within normal limits, including C-reactive protein (CRP), leucocyte counts and C1-esterase inhibitor. Allergies, concomitant diseases, preceding acute infections, urticaria and fever were denied.
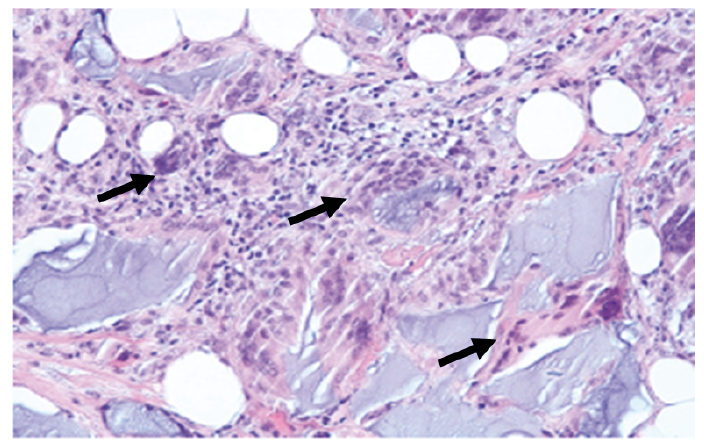
A biopsy specimen from the cheek revealed dermal and subcutaneous irregular-shaped basophilic material, morphologically corresponding more to hyaluronic acid (HA) (5) than to PMMA, which was surrounded by a granulomatous foreign body reaction consisting of numerous multinucleated giant cells, histiocytes and lymphocytic infiltrates accompanied by strong proliferation of fibrous septa (Fig. 2).
Magnetic resonance imaging revealed a widespread oedematous inflammatory process with granulomatous nodule formation and extensive repression of subcutaneous fatty tissue, particularly in the middle and lateral malar regions (Fig. 3).
As local therapies were not feasible due to the diffuse inflammation in the absence of demarcated nodules, a short course of oral methylprednisolone was initiated (initial dosage 1 mg/kg bodyweight), which led to a marked decrease in oedema and pain within a few days (Fig. 1B), but prompt reoccurrence on dose reduction. Following a proposed therapy algorithm for nodular foreign body reactions with LIRS, systemic methylprednisolone was reinitiated, combined with oral clindamycin (300 mg b.i.d.) and ciprofloxacin (500 mg t.i.d.) for 4 weeks (4). In an effort to reduce glucocorticoid intake, hydroxychloroquine (200 mg/day) was subsequently added, but failed to prevent another flare-up of symptoms upon methylprednisolone tapering. While swelling and oedema were stably reduced under subsequent maintenance therapy with dimethyl fumarate (DMF) (120 mg/day) and low-dose prednisolone (5 mg/day), DMF had to be discontinued due to related side-effects (i.e. headache and diarrhoea).
The pathophysiology underlying the transition from a limited physiological foreign body reaction, which contributes to the desired cosmetic “feeding effect” of injectable fillers, such as HA, for volumetric rejuvenation (6), to severe and chronic inflammatory processes is complex and incompletely understood (4). In chronic granulomatous foreign body reaction, persistent unsuccessful phagocytosis stimulates macrophage activation along with a pro-inflammatory Th1 cytokine pattern (i.e. TNF-α, INF-γ, IL-12) (6). Delayed immune-mediated adverse events, such as LIRS, have been postulated to be triggered by microbial biofilm colonization of the filler material (4), in which bacterial cell wall components may foster a milieu of permanent immune activation, possibly by binding to monocyte toll-like receptors (TLR) by molecular mimicry (4, 6). While a predisposing genetic background seems likely, non-specific systemic infections, vaccinations or unspecific trauma of the injection site have been reported to commonly precede late inflammatory reactions in patients with bioimplants (4, 7).
Filler removal, excision or intralesional administration of glucocorticosteroids or enzyme (e.g. hyaluronidase) may be beneficial for solitary granulomatous nodules, but therapeutic options for widespread granuloma formation and late-onset inflammation after dermal filler injections are scarce (4). Although not licensed for this use, antimalarials, such as hydroxychloroquine, have been proposed for the treatment of delayed immune-mediated adverse effects of dermal fillers, as they may inhibit TLR-mediated inflammation by decreasing lipopolysaccharide- and CpG-induced IL-6 expression by monocyte (8). In non-infectious granulomatous skin diseases, the efficacy of fumaric acid esters has been attributed to antiproliferative effects mediated by TNF-induced NF-κB binding and induction of a switch towards a Th2-polarized cytokine milieu (9).
Further studies are required to systematically investigate the safety and efficacy of DMF and other potential therapeutic approaches for late inflammatory complications after dermal filler injection. To date, despite a continuously increasing demand for aesthetic contouring and rejuvenation with soft-tissue fillers (10) the basis for complication diagnosis and management remains restricted mostly to expert opinion and casuistic reports with limited data from controlled clinical trials. The current case draws attention to the demand for long-term safety data and evidence-based treatment recommendations, particularly for late complications of soft tissue fillers in aesthetic medicine.
The authors thank the patient for granting permission to publish this information.