ORIGINAL REPORT
Efficacy and Safety of Tildrakizumab in Older Patients: Pooled Analyses of Two Randomized Phase III Clinical Trials (reSURFACE 1 and reSURFACE 2) Through 244 Weeks
Elke L. M. TER HAAR1, Juul M. P. A. VAN DEN REEK1, Kristian GAARN DU JARDIN2, Almudena BARBERO-CASTILLO2, Elke M. G. J. DE JONG1 and Satish F. K. LUBEEK1
1Department of Dermatology, Radboud University Medical Centre, Nijmegen, The Netherlands and 2Almirall R&D, Barcelona, Spain
The evidence on treating older patients with psoriasis with modern biologics is scarce. This study compared the efficacy and safety of tildrakizumab among younger and older patients with psoriasis (< 65/≥ 65 years) in a post hoc analysis of 2 phase III trials (reSURFACE1/2, n = 1,862). Tildrakizumab 100 mg/200 mg was administered at weeks 0/4/every 12 weeks thereafter. At week 28, patients with ≥ 75% improvement in baseline Psoriasis Area and Severity Index (PASI75) in reSURFACE1 were re-randomized to the same tildrakizumab dose or placebo; in reSURFACE2, PASI75 responders to 200 mg were re-randomized to tildrakizumab 100 mg or 200 mg; PASI75 responders to 100 mg maintained their dose. At weeks 64/52 (reSURFACE1/2), PASI50 responders entered an extension period (weeks 256/244). Outcomes were proportion of patients with PASI < 3, Dermatology Life Quality Index (DLQI) 0/1, comorbidities, comedication, and side-effects. The proportion of patients with a PASI < 3 was similar and maintained (tildrakizumab 100 mg and 200 mg, week 244: 83.3% and 84.1%/92.3% and 100.0%); DLQI 0/1 proportions at week 52 were 66.8% and 72.0%/68.3% and 81.3%. Comorbidity and comedication were more common in older patients. The safety profile of tildrakizumab appeared favourable in both groups. Tildrakizumab in patients ≥ 65 years appears effective and safe in long-term psoriasis management. These findings might assist treatment selection and overcome treatment reluctance.
SIGNIFICANCE
This study compared the efficacy and safety of tildrakizumab among younger and older adults with psoriasis (< 65/≥ 65 years). High and similar proportions of patients in both groups achieved improvement of skin lesions and disease-related quality of life during the first year, which was maintained up to 5 years. The most frequent side-effect was nasopharyngitis. Although older patients presented more comorbidities and comedication, they showed a similar and favourable safety profile, demonstrating that tildrakizumab appears to be a good and safe treatment for psoriasis in both older and younger patients with psoriasis.
Key words: adverse drug events; clinical efficacy; clinical trials, Phase III as topic; older adults, frail; tildrakizumab.
Citation: Acta Derm Venereol 2023: 103: adv17752. DOI: https://doi.org/10.2340/actadv.v103.17752.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Sep 19, 2023; Published: Oct 25, 2023
Corr: Elke L. M. ter Haar, Department of Dermatology, Radboud University Medical Centre, Post Office Box 9101, NL-6500 HB Nijmegen, The Netherlands. E-mail: Elke.terHaar@radboudumc.nl
Competing interests and funding: EtH has carried out investigator-initiated research with financial support from Almirall and has carried out clinical trials for Novartis. All funding is not personal but goes to the independent Research Fund of the Department of Dermatology of Radboud University Medical Centre Nijmegen (Radboudumc), The Netherlands; JvdR carried out clinical trials for AbbVie, Celgene and Janssen and has received speaking fees/attended advisory boards from AbbVie, Janssen, BMS, Almirall, Leo Pharma, Novartis, UCB and Eli Lilly and reimbursement for attending a symposium from Janssen, Pfizer, Celgene and AbbVie. All funding is not personal but goes to the independent research fund of the Department of Dermatology of Radboudumc Nijmegen, the Netherlands; KGdJ and ABC are employers of Almirall; EdJ has received research grants for the independent research fund of the Department of Dermatology of Radboudumc Nijmegen, the Netherlands from AbbVie, BMS, Janssen, Leo Pharma, Novartis, and UCB for research on psoriasis. Has acted as consultant and/or paid speaker for and/or participated in research sponsored by companies that manufacture drugs used for the treatment of psoriasis or eczema including AbbVie, Amgen, Almirall, Celgene, Galapagos, Janssen, Lilly, Novartis, Leo Pharma, Sanofi and UCB. All funding is not personal but goes to the independent research fund of the Department of Dermatology of Radboudumc Nijmegen, the Netherlands; SL has received research grants for investigator-initiated research from Almirall and has acted as consultant, advisory board member and/or paid speaker for Leo Pharma, Sanofi Genzyme, Sunpharma, Almirall, and Janssen. All financial compensations/grants were paid to the independent research fund of the Department of Dermatology of Radboudumc Nijmegen, the Netherlands.
This publication was funded by Almirall R&D, Barcelona, Spain.
INTRODUCTION
Psoriasis is a chronic inflammatory disease with a worldwide prevalence rate of approximately 2–3% (1). Older patients (age ≥ 65 years) represent an increasing proportion of patients with psoriasis and 15% of them have moderate to severe disease (2). With a steadily ageing population (3), physicians are faced with an increasing number of older patients with psoriasis. However, optimal treatment selection might be difficult due to the presence of comorbidities (4), comedication (5, 6), and adverse events (AEs) (7), which also influence patient treatment preferences (8).
Generally, biologics have demonstrated even better efficacy than conventional systemics (9, 10), with lower rates of AE than conventional systemics (10). However, the elderly population is often excluded from clinical trials based on age or on age-related factors (e.g. comorbidities) (11), and representation of older patients in the available trial literature is low. However, a recent registry reported that discontinuation of biologics due to AEs did not occur more frequently in older compared with younger patients (12). Older patients tended to have more serious infections, non-melanoma skin cancer (NMSC) and malignancies than younger patients, possibly due to the ageing process and more extensive duration of disease (9, 13).
In the case of tildrakizumab (TIL), specifically, there is almost no evidence available regarding older patients, and the first report in clinical practice was provided by Ruggiero et al. (14). This study included only 6 older patients, but they reported similar results to those of randomized clinical trials (15). Although biologics seem to be relatively safe, this limited evidence-based management could trigger treatment reluctance to prescribe (newer) biologics in older patients for fear of lower efficacy or worse tolerability. Thus, more robust, comprehensive data regarding biologics in older patients are needed.
The aim of the present study is to compare the pooled efficacy and safety of TIL 100 mg and 200 mg for 244 weeks among younger and older patients from the 2 pivotal reSURFACE trials (reSURFACE 1 and reSURFACE 2) (16), including long-term extension periods (17, 18).
MATERIALS AND METHODS
This is a post hoc pooled analysis of 2 3-part, randomized, double-blind, placebo-controlled, parallel-group, phase III trials (reSURFACE 1 and reSURFACE 2, ClinicalTrials.gov NCT01722331 and NCT01729754) that evaluated the efficacy and safety of TIL in patients with moderate to severe chronic plaque psoriasis for up to 5 years (17, 18). reSURFACE 2 included etanercept as an active comparator (16). reSURFACE 1 was conducted from 10 December 2012 to 28 October 2015. reSURFACE 2 was conducted from 12 February 2013 to 28 September 2015.
Main interventions
The main inclusion and exclusion criteria at baseline were similar between trials. Baseline study inclusion and exclusion criteria, patient characteristics, treatment, and methodology of these 2 pivotal clinical trials have been reported previously (16, 18). A total of 1,862 patients ≥ 18 years with moderate to severe chronic plaque psoriasis diagnosed ≥ 6 months prior to enrolment, with a body surface area ≥ 10%, a Physician’s Global Assessment ≥ 3 and a Psoriasis Area and Severity Index (PASI) ≥ 12, were included (reSURFACE 1, n = 772; reSURFACE 2, n = 1,090) (16). In reSURFACE 1, patients were randomized to TIL 100 mg, 200 mg or placebo (2:2:1). In reSURFACE 2, patients were randomized to TIL 100 mg, 200 mg, placebo or etanercept 50 mg (2:2:1:2). Tildrakizumab was administered at weeks 0, 4 and every 12 weeks afterwards. Responders were defined as patients with ≥ 75% improvement in baseline PASI (PASI75). At week 28, PASI75 responders in reSURFACE 1 were re-randomized to continue with the same TIL dose or to receive placebo; in reSURFACE 2, PASI75 responders to TIL 200 mg were re-randomized to TIL 100 mg or 200 mg, while PASI75 responders to TIL 100 mg maintained the same dose. At week 64 (reSURFACE 1) or week 52 (reSURFACE 2), patients with ≥ 50% improvement from baseline PASI score entered an optional 192-week extension period, until week 256 (reSURFACE 1) or week 244 (reSURFACE 2) (17, 18).
Both reSURFACE trials were conducted in accordance with Good Clinical Practice guidelines and the principles of the Declaration of Helsinki 1964, and its successive amendments. The study protocols received local institutional review board or ethics committee approvals. All patients gave informed consent to participate in the trials.
Main outcome measures
Medical history, including comorbidities and comedications, for each age group were summarized with descriptive statistics. Main efficacy outcomes were defined as the proportion of patients achieving absolute PASI < 3 over 5 years of treatment; that is, at weeks 28, 52 and 244, and Dermatology Life Quality Index (DLQI)/DLQI-Relevant (DLQI-R) 0/1 responses at weeks 28 and 52. In the DLQI, non-relevant responses (NRR) are scored as having no impact on patient quality of life, artificially improving patients’ DLQI scores (19). The new DLQI-R scoring avoids the bias of the NRR option by adjusting the total score for relevant items (20). Proportions of patients achieving absolute PASI < 5 and < 1 were also evaluated. Analyses were stratified by age groups and TIL dose, attending to the following groups: < 65 years and ≥ 65 years, TIL 100 mg and 200 mg.
Safety assessments included a description of AEs. Pre-specified treatment-emergent AEs (TEAEs) comprised severe infections, malignancies, NMSC, melanoma, confirmed extended major adverse cardiovascular events (MACE), injection site reaction and drug-related hypersensitivity reactions (16, 18). Adverse events were assessed at all study visits and classified according to age and dose split. Preferred terms from the Medical Dictionary for Regulatory Activities for each AE were assigned to the treatment dose that the patient was actively receiving when the AE occurred.
Statistical analysis
Current post hoc analyses focus on differences between age groups in demographics (including comorbidities and comedications), absolute PASI response, DLQI and DLQI-R response (by adjusting the total questionnaire score by the number of NNRs indicated by a patient) (21), and safety.
No formal hypothesis testing was performed for these post hoc analyses. All subjects randomized to TIL 100 mg and 200 mg who received at least 1 dose of study medication were included for week 28 efficacy analyses (TIL 100 mg: n = 593, 541 patients aged < 65 years and 52 patients aged ≥ 65 years; TIL 200 mg: n = 597, 547 patients aged < 65 years and 50 patients aged ≥ 65 years) (Fig. 1). All patients who were responders (i.e. PASI75) at week 28 and who continued treatment with the same TIL dose were included for the long-term efficacy analyses (weeks 52 and 244) (TIL 100 mg: n = 329, 303 patients aged < 65 years and 26 patients aged ≥ 65 years; TIL 200 mg: n = 227, 211 patients aged < 65 years and 16 patients aged ≥ 65 years) (Fig. 1).
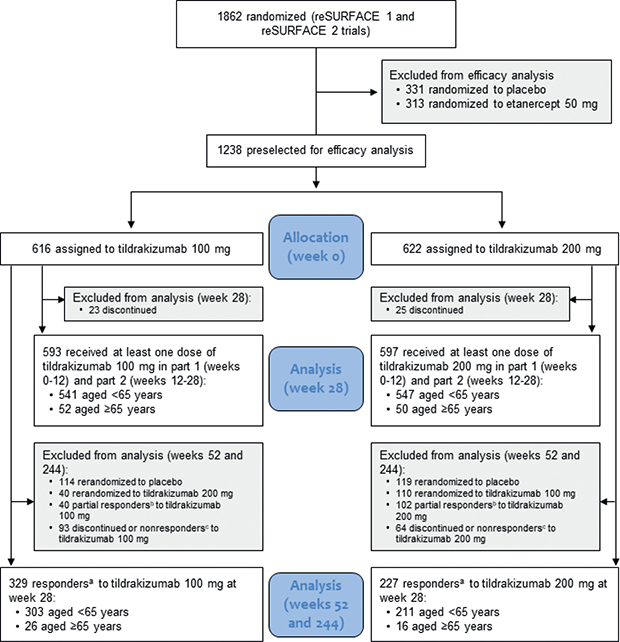
Fig. 1. Patient disposition. aPatients with ≥ 75% improvement in Psoriasis Area and Severity Index (PASI); bpatients with ≥ 50 to < 75% improvement in PASI; cpatients with < 50% improvement in PASI.
Efficacy analyses used an observed case approach. A multiple imputation approach (10 imputations) was used for missing data as sensitivity analyses for the PASI outcome, as described previously (18).
A mixed model was performed in the observed case population to evaluate possible changes in the absolute PASI at weeks 28 and 244 according to the following independent factors: age group, treatment, week, prior biological therapy for psoriasis, smoking habit, diabetes mellitus, history of psoriatic arthritis. We also included the age group x week interaction term into the model (looking at whether the behaviour of the variable under study in the 2 age groups is different over time, regardless of treatment). The model was covaried by baseline PASI and body mass index (BMI). This analysis was repeated with a multiple imputation approach.
Analyses of comorbidities and comedications were performed in all randomized patients (n = 1,190). Concomitant medications were collected over the 5-year study period. Safety analyses were performed in all patients who received at least 1 dose of study drug by treatment received (n = 1,800). Safety data from week 0 to 5 years were pooled between reSURFACE 1 (up to week 256) and reSURFACE 2 (up to week 244) and presented for patients who received TIL during any part of the study with age of 65 years as the comparison threshold. Safety data are reported as number of events per 100 patient-years of exposure; exposure-adjusted incidence rates and 95% confidence intervals (95% CIs) were calculated as described previously (16, 18).
Analyses were performed with SAS software, version 9.4 (TS1M7) (© 2016 by SAS Institute INC, Cary, NC, USA), on the X64_10PRO platform for Windows, extension package SAS/STAT® software, version 15.2.
RESULTS
Demographic and baseline characteristics
A summary of baseline characteristics is shown in Table I. The percentage of women was slightly higher in the older vs younger group and baseline DLQI score was significantly lower in older vs younger patients (p = 0.002). The median (range) age for each age group was 44.0 (18.0–64.0) years in the younger, and 68.0 (65.0–82.0) years in the older group (Fig. S1). Both age groups showed no differences in previous experience with systemic biologic or non-biologic treatments.
| < 65 years | ≥ 65 years | p-valuec | |||||
| 100 mg (n = 541) | 200 mg (n = 547) | Total (n = 1,088) | 100 mg (n = 52)a | 200 mg (n = 50)b | Total (n = 102) | ||
| Age, years, n, mean (SD) | 541, 43.2 (11.3) | 547, 43.8 (11.9) | 1,088, 43.5 (11.6) | 52, 70.2 (4.6) | 50, 68.4 (3.6) | 102, 69.3 (4.3) | NA |
| Female, n (%) | 161/541 (29.8) | 142/547 (26.0) | 303/1,088 (27.9) | 19/52 (36.5) | 19/50 (38.0) | 38/102 (37.3) | 0.04 |
| BMI, kg/m2, n, mean (SD) | 540, 30.0 (7.1) | 547, 29.8 (7.5) | 1,087, 29.9 (7.3) | 51, 31.4 (7.5) | 50, 30.1 (7.5) | 101, 30.7 (7.5) | 0.24 |
| Weight, kg, n, mean (SD) | 541, 89.1 (23.1) | 547, 89.1 (23.2) | 1,088, 89.1 (23.1) | 52, 91.2 (23.1) | 50, 85.0 (17.8) | 102, 88.2 (20.8) | 0.70 |
| PASI score, n, mean (SD) | 541, 20.2 (7.9) | 547, 20.3 (8.0) | 1,088, 20.2 (8.0) | 52, 18.9 (6.2) | 50, 19.8 (8.5) | 102, 19.4 (7.4) | 0.29 |
| BSA, (%), n, mean (SD) | 541, 31.7 (18.4) | 542, 31.3 (17.4) | 1,083,31.5 (17.9) | 52, 29.0 (13.2) | 50, 32.4 (19.1) | 102, 30.7 (16.3) | 0.65 |
| DLQI score, n, mean (SD) | 538, 14.4 (7.0) | 541, 13.5 (7.0) | 1,079, 13.9 (7.0) | 52, 12.8 (7.6) | 50, 10.4 (6.1) | 102, 11.7 (6.9) | 0.002 |
| PsA, n (%) | 89/541 (16.5) | 87/547 (15.9) | 176/1,088 (16.2) | 9/52 (17.3) | 11/50 (22.0) | 20/102 (19.6) | 0.37 |
| PGA ≥ 4, n (%) | 177/541 (32.7) | 185/547 (34.0) | 362/1,088 (33.4) | 16/52 (30.8) | 14/50 (28.0) | 30/102 (29.4) | 0.42 |
| Previous experience with systemic biologic treatment, n (%) | 94/541 (17.4) | 95/547 (17.4) | 189/1,088 (17.4) | 11/52 (21.2) | 11/50 (22.0) | 22/102 (21.6) | 0.29 |
| Previous experience with systemic non-biologic treatment, n (%)d | 174/541 (32.1) | 194/547 (35.5) | 368/1,088 (33.8) | 16/52 (30.8) | 10/50 (20.0) | 26/102 (25.5) | 0.09 |
| an = 26 after W28 re-randomization; bn = 16 after W28 re-randomization; cStudents t-tests are used for numerical variables and χ2 tests for categorical data. Comparisons involve the 2 total age groups; dexcluding phototherapy. When presenting percentages, numerator and denominator are reported. BMI: body mass index; BSA: body surface area; DLQI: Dermatology Life Quality Index; NA: not applicable; PASI: Psoriasis Area and Severity Index; PGA: Physician Global Assessment; PsA: psoriatic arthritis; SD: standard deviation; w: week. | |||||||
The most common comorbidities in patients < 65 years vs ≥ 65 years were musculoskeletal and connective tissue disorders (26.4% vs 43.1%, p < 0.001), metabolic and nutrition disorders (26.0% vs 56.9%, p < 0.001), vascular disorders (24.4% vs 70.6%, p < 0.001), and immune system disorders (22.3% vs 20.6%, p = 0.80). The complete medical history, with comorbidities, by age group is shown in Table SI.
The proportions of patients < 65 years vs ≥ 65 years taking comedication at baseline were 56.3% vs 87.3%. Table SII shows comedication reported by patients over the 5-year study period.
Efficacy outcomes
PASI score. Fig. 2 shows the absolute mean PASI score over time by age group and TIL dose. Throughout the first 28 weeks, and especially between weeks 0 and 8, a decrease in the absolute mean PASI was observed in the 2 age groups and for each dose, from mean scores of ≥ 19 at baseline to means ≤ 6 from week 12. This trend was then maintained until week 244.
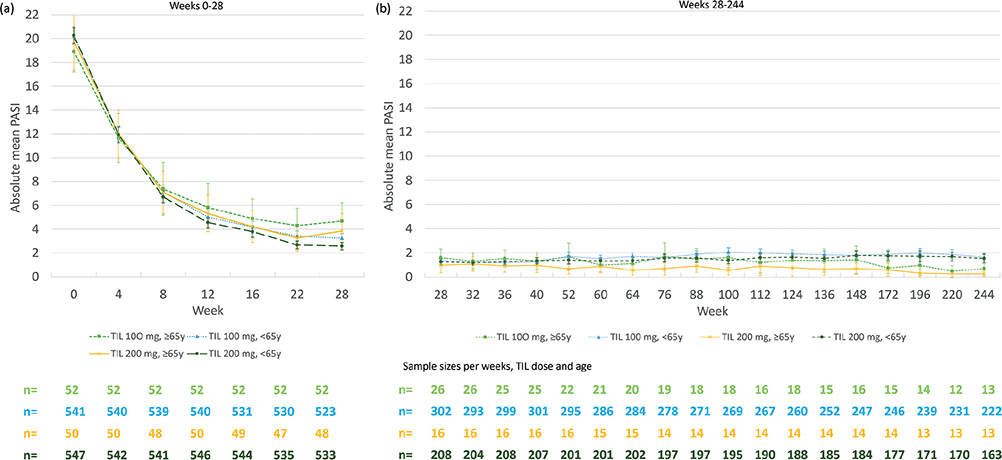
Fig. 2. Mean of absolute Psoriasis Area and Severity Index (PASI) by age group and tildrakizumab (TIL) dose over time (observed case approach). (a) 0–28 weeks, (b) 28–244 weeks. Bars indicate 95% confidence intervals (95% CI). The number of patients with available PASI scores over time are shown below the graphs. y: years.
The proportion (95% CI) of TIL-treated patients aged < 65 vs ≥ 65 years achieving an absolute PASI < 3 for TIL 100 mg at week 28 was 66.4% (62.1–70.4%) vs 51.9% (37.6–66.0%). At week 244, it was 83.3% (77.8–88.0%) vs 92.3% (64.0–99.8%). The proportion (95% CI) of TIL-treated patients aged < 65 vs ≥ 65 years achieving an absolute PASI < 3 for TIL 200 mg at week 28 was 70.4% (66.3–74.2%) vs 58.3% (43.2–72.4%). At week 244, it was 84.1% (77.5–89.3%) vs 100.0% (75.3–100.0%) (comparison by age groups (combining TIL doses) week 244: p = 0.09).
The proportions of patients with PASI < 5 coincided with those found for PASI < 3, with no differences between age groups at week 244. The proportions of patients with PASI < 1 was lower compared with PASI < 3 and < 5, with a slight tendency to show a benefit in patients aged ≥ 65 vs < 65 years at week 244 (see Appendix S1).
Absolute mean PASI and absolute PASI < 3, < 5 and < 1 results evaluated by the sensitivity analysis (multiple imputation) is shown in Fig. S2 and Appendix S2, respectively.
There was no effect of age group on absolute PASI at weeks 28 and 244. There was a significant effect of baseline PASI, treatment, week and smoking status (p < 0.001) on absolute PASI at week 28. At week 244, there were significant effects on absolute PASI for baseline PASI (p < 0.001), treatment (p = 0.02), BMI (p = 0.001), prior biological therapy for psoriasis (p = 0.01), smoking status (p = 0.007), and the interaction term age group × week (p = 0.003) (Table SIII). The mixed model on PASI course for sensitivity analysis with multiple imputation is shown in Table SIV.
DLQI and DLQI-R. The proportion (95% CI) of TIL-treated patients aged < 65 vs ≥ 65 years achieving a DLQI 0/1 for TIL 100 mg at week 28 was 53.8% (49.5–58.2%) vs 53.9% (39.5–67.8%), and at week 52, it was 66.8% (61.1–72.1%) vs 68.2% (45.1–86.1%). The proportion (95% CI) of TIL-treated patients aged < 65 vs ≥ 65 years achieving a DLQI 0/1 for TIL 200 mg at week 28 was 61.1% (56.8–65.3%) vs 60.4% (45.3–74.2%), and at week 52 it was 72.0% (65.2–78.1%) vs 81.3% (54.4–96.0%) (see Fig. 3) (comparison by age groups (combining TIL doses) at week 52: p = 0.54).
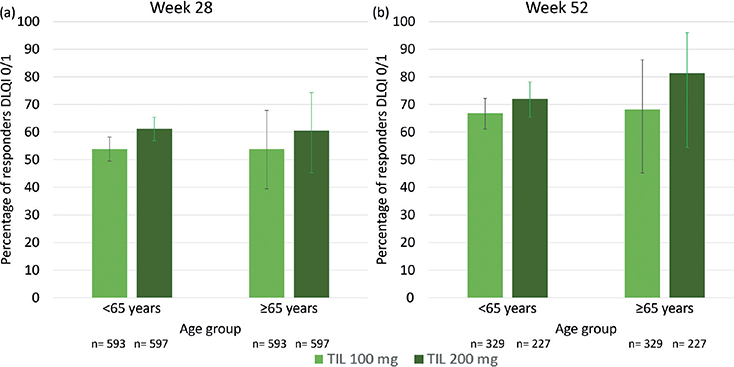
Fig. 3. Percentage of patients achieving Dermatology Life Quality Index (DLQI) 0/1 by age group and tildrakizumab (TIL) dose at weeks 28 and 52 (observed case approach). Bars indicate the 95% confidence interval (95% CI).
The absolute mean DLQI scores and DLQI-R results are shown in Appendix S3. The mean absolute DLQI and DLQI-R scores were similar, except for patients aged ≥ 65 years for TIL 100 mg at week 28, where the mean DLQI-R was higher (4.5 (5.2) vs 3.4 (3.9)). The proportions of patients for each age group maintained the same trend for DLQI-R as those observed for DLQI, although the proportions are lower for the former.
Safety outcomes
Summary of exposure adjusted rates of AEs attending TIL dose and age are shown in Table II. The cumulative incidence of TEAEs for TIL 100 mg/TIL 200 mg in patients < 65 vs ≥ 65 years was 4,717/5,032 vs 515/545 per 100 patient-years of exposure, within which the highest cumulative incidence of infections were 621/592 vs 23/51 per 100 patient-years of exposure for nasopharyngitis, followed by 168/203 vs 11/10 for other upper respiratory tract infection, and 76/94 vs 3/10 for influenza.
| < 65 years | ≥ 65 years | |||
| 100 mg (n = 793) | 200 mg (n = 846) | 100 mg (n = 79) | 200 mg (n = 82) | |
| Total follow-up, patient-year | 2,487.7 | 2,531.6 | 200.7 | 221.9 |
| Any SAE | 205 (8.2) [7.1–9.4] | 206 (8.1) [7.0–9.3] | 44 (21.9) [15.3–28.5] | 39 (17.6) [12.0–23.2] |
| Drug-related SAEs | 18 (0.7) [0.4–1.1] | 11 (0.4) [0.2–0.7] | 6 (3.0) [0.6–5.4] | 5 (2.3) [0.2–4.3] |
| SAEs leading to discontinuation | 22 (0.9) [0.5–1.3] | 16 (0.6) [0.3–1.0] | 9 (4.5) [1.5–7.5] | 7 (3.2) [0.8–5.5] |
| Drug-related SAEs leading to discontinuation | 6 (0.2) [0.0–0.4] | 4 (0.2) [0.0–0.3] | 3 (1.5) [0.0–3.2] | 1 (0.5) [0.0–1.4] |
| Any TEAE | 4,717 (189.6) [184.1–195.1] | 5,032 (198.8) [193.2–204.4] | 515 (256.6) [234.0–279.2] | 545 (245.6) [224.6–266.7] |
| Drug-related TEAEs | 752 (30.2) [28.1–32.4] | 989 (39.1) [36.6–41.6] | 41 (20.4) [14.1–26.8] | 51 (23.0) [16.6–29.4] |
| TEAEs leading to discontinuation | 42 (1.7) [1.2–2.2] | 30 (1.2) [0.8–1.6] | 10 (5.0) [1.8–8.1] | 10 (4.5) [1.7–7.4] |
| Drug-related AEs leading to discontinuation | 16 (0.6) [0.3–1.0] | 8 (0.3) [0.1–0.5] | 3 (1.5) [0.0–3.2] | 2 (0.9) [0.0–2.2] |
| Deaths | 9 (0.446) [0.1–0.6] | 4 (0.2) [0.0–0.3] | 2 (1.0) [0.0–2.4] | 1 (0.5) [0.0–1.4] |
| TEAEs of special interest: | ||||
| Severe infectiona | 31 (1.3) [0.8–1.7] | 41 (1.6) [1.1–2.1] | 7 (3.5) [0.9–6.1] | 7 (3.2) [0.8–5.5] |
| Malignancy excluding NMSC | 17 (0.7) [0.4–1.0] | 11 (0.4) [0.2–0.7] | 4 (2.0) [0.0–4.0] | 6 (2.7) [0.5–4.9] |
| NMSC | 6 (0.2) [0.0–0.4] | 10 (0.4) [0.2–0.6] | 8 (4.0) [1.2–6.8] | 6 (2.7) [0.5–4.9] |
| Confirmed extended MACE | 14 (0.6) [0.3–0.9] | 21 (0.8) [0.5–1.2] | 1 (0.5) [0.0–1.5] | 3 (1.4) [0.0–2.9] |
| Injection-site reaction | 66 (2.7) [2.0–3.3] | 81 (3.2) [2.5–3.9] | 1 (0.5) [0.0–1.5] | 5 (2.3) [0.2–4.3] |
| Drug-related hypersensitivity reaction | 14 (0.6) [0.3–0.9] | 5 (0.2) [0.0–0.4] | 0 | 0 |
| Data shown as n (number of events per 100 patient-years of exposure) [95% CI]. aSevere infection was defined as any infection meeting the regulatory definition of a serious AE (i.e. resulted in death, was life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, or required intervention to prevent 1 of the other outcomes listed), or any infection requiring intravenous antibiotic. 95% CI: 95% confidence interval; MACE: major adverse cardiovascular event; NMSC: non-melanoma skin cancer; SAEs: serious AEs. | ||||
With regards to TEAEs of special interest, the cumulative incidence for TIL 100 mg/TIL 200 mg in patients < 65 years vs patients ≥ 65 years was 17/11 vs 4/6 per 100 patient-years of exposure for malignancy excluding NMSC, 6/10 vs 8/6 for NMSC, and 14/21 vs 1/3 for confirmed extended MACEs.
A total of 6 (0.2%) drug-related serious AEs (SAEs) per 100 patient-years of exposure (100 mg TIL)/4 (0.2%) (200 mg TIL) led to discontinuation in the younger group, and 3 (12.5%)/1 (0.5%) in the older group. The specific drug-related SAEs are shown in Table III.
DISCUSSION
The increasing number of elderly patients (≥ 65 years) with moderate to severe psoriasis in daily practice represents a challenge for dermatologists. However, evidence in this patient population is limited to a few biological agents and small-molecule inhibitors (22). This is one of the first studies to depict the efficacy and safety data of TIL for older vs younger patients from randomized clinical trials. This comparison is important because of possible differences in patient profile and the increasing number of older patients with psoriasis needing a safe and effective treatment. TIL in patients ≥ 65 years appears to be effective and safe in long-term psoriasis management, which was comparable to younger patients.
Differences in comorbidities were evident between both age groups. The current study found a higher proportion of musculoskeletal, metabolic, and vascular disorders, proportionally, in older patients. These disorders are more common in old age (23–25), and have been (partially) related to the existence of psoriasis (26–28). Biologics appear to have a good safety profile and are usually well tolerated. In addition, in terms of comedication, older patients had a higher intake of drugs related to cardiac or gastric problems. Since TIL is cleared from the body by general protein catabolism processes, and is not eliminated by renal or hepatic pathways, no interaction between TIL and the comedications taken in this population has been described (29).
Although the current study found some differences among the presence of comorbidities and comedication, comparable long-term PASI and DLQI responses were found in younger and older patients, independently of the administration dose, without safety concerns. In the long-term (week 244), the current study found that more than 80% and 90% of younger and older patients, respectively, showed a PASI < 3. These results are consistent with other studies on the long-term effects of different biologics on PASI responses (30).
The proportion of subjects with at least 1 NRR in the DLQI was higher for the older group, which also showed a significantly lower baseline DLQI level. Non-relevant responses on the DLQI may be associated with an underestimation of disease severity (31). In patients with psoriasis who marked 1 or more NRRs, the DLQI-R seems more sensitive compared with the DLQI (32), with the rates of patients with psoriasis with NRRs being higher for older patients (33). The current study showed that the proportions of patients with DLQI-R 0/1 were similar and/or slightly lower compared with the DLQI 0/1. In this sense, the improved measurement properties of the new DLQI-R score in psoriasis are well established (21, 34–36).
Safety analysis showed a favourable tolerability profile in both age groups. Adverse events were consistent with the rates observed in other clinical trials with biologics (37). In terms of infection, both age groups showed a similar profile, sharing the highest incidence of nasopharyngitis and other respiratory tract infection, in line with those of phase II–III trials with biologics (38, 39) and available real-world evidence (RWE) registries (40), with no new safety evidence. However, in terms of TEAEs of special interest, older patients showed proportionally more cases of cardiovascular events, NMSC and other malignancies compared with younger patients, as previously described in the literature (13), most likely related to the ageing process and the longer psoriatic disease duration, and not due to the psoriatic treatment administered. In general, these results demonstrate the potential benefit of TIL in older patients without affecting their safety profile.
Limitations
This study has some limitations. The main limitation is the relatively small number of older patients. In addition, older patients with extensive multimorbidity and/or polypharmacy are less likely to be included in clinical trials. The investigated older patients may still be a relatively healthy older group, as they passed the clinical trial inclusion and exclusion criteria. In this vein, exclusion criteria for reSURFACE trials are not based on (or represent) RWE. For example, some of the common comorbidities associated with psoriasis or its variants (psoriatic arthritis, erythrodermic psoriasis) were exclusion criteria in the reSURFACE studies, as well as recurrent infections (41–43). In addition, patients with extensive pre-treatment were excluded, as they had to wait until their psoriasis showed a PASI ≥ 12. In clinical practice, this is not feasible, under-representing to the trial populations what clinicians see in daily clinical practice. Despite the fact that some data indicate that patients treated in routine practice with TIL differed substantially from those included in phase III studies (44), a RWE study has recently confirmed that there is no efficacy-effectiveness gap for TIL (45). Further research with a larger number of older patients in a real-world setting is needed to confirm these preliminary results. Another limitation is the lack of age-randomized groups and control settings.
Conclusion
In these current post hoc analyses, TIL demonstrated long-term control with a favourable safety in patients below and above 65 years of age. This confirms the limited RWE on the clinical effectiveness of TIL in older patients with moderate to severe psoriasis. Despite the differences between the age groups in terms of comorbidities and comedications, these results indicate a similar percentage improvement in disease severity in the 2 age groups, with comparable improvements in quality of life and without major safety issues. Further confirmatory studies are desirable, with dedicated and real-world trials to better understand the profile of biological management of this group of patients.
ACKNOWLEDGEMENTS
Medical writing assistance in the preparation of this manuscript was provided by Mónica Giménez, PhD, and Eva Mateu, PhD, of TFS HealthScience.
REFERENCES
- Sewerin P, Brinks R, Schneider M, Haase I, Vordenbäumen S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis 2019; 78: 286–287.
- Kwon HH, Kwon IH, Youn JI. Clinical study of psoriasis occurring over the age of 60 years: is elderly-onset psoriasis a distinct subtype? Int J Dermatol 2012; 51: 53–58.
- NIH. World’s older population grows dramatically. National Institute on Aging. [Accessed 2022 July 22]. Available from: https://www.nia.nih.gov/news/worlds-older-population-grows-dramatically.
- Phan C, Sigal ML, Estève E, Reguiai Z, Barthélémy H, Beneton N, et al. Psoriasis in the elderly: epidemiological and clinical aspects, and evaluation of patients with very late onset psoriasis. J Eur Acad Dermatol Venereol 2016; 30: 78–82.
- Pérez-Jover V, Mira JJ, Carratala-Munuera C, Gil-Guillen VF, Basora J, López-Pineda A, et al. Inappropriate use of medication by elderly, polymedicated, or multipathological patients with chronic diseases. Int J Environ Res Public Health 2018; 15: 310.
- Banerjee A, Mbamalu D, Ebrahimi S, Khan AA, Chan TF. The prevalence of polypharmacy in elderly attenders to an emergency department – a problem with a need for an effective solution. Int J Emerg Med 2011; 4: 22.
- Davies EA, O’Mahony MS. Adverse drug reactions in special populations – the elderly. Br J Clin Pharmacol 2015; 80: 796–807.
- van Winden MEC, Ter Haar ELM, Groenewoud JMM, van de Kerkhof PCM, de Jong EMGJ, Lubeek SFK. Quality of life, treatment goals, preferences and satisfaction in older adults with psoriasis: a patient survey comparing age groups. Br J Dermatol 2021; 184: 759–762.
- Ricceri F, Bardazzi F, Chiricozzi A, Dapavo P, Ferrara F, Mugheddu C, et al. Elderly psoriatic patients under biological therapies: an Italian experience. J Eur Acad Dermatol Venereol 2019; 33: 143–146.
- Garber C, Plotnikova N, Au S Chung, Sorensen EP, Gottlieb A. Biologic and conventional systemic therapies show similar safety and efficacy in elderly and adult patients with moderate to severe psoriasis. J Drugs Dermatol 2015; 14: 846–852.
- Schaap MJ, van Winden MEC, Seyger MMB, de Jong EMGJ, Lubeek SFK. Representation of older adults in randomized controlled trials on systemic treatment in plaque psoriasis: a systematic review. J Am Acad Dermatol 2020; 83: 412–424.
- Haar ELM ter, Thomas SE, van den Reek JMPA, Otero ME, Njoo MD, Ossenkoppele PM, et al. Drug survival, safety, and effectiveness of biologics in older patients with psoriasis: a comparison with younger patients – a BioCAPTURE Registry study. Drugs Aging 2022; 39: 715–727.
- Haar ELM ter, Bruin EE ten, Bronkhorst EE, Borgonjen RJ, Kleinpenning MM, Kop EN, et al. Safety assessment of conventional and biological systemic therapy in older adults with psoriasis, a real-world multicentre cohort study. Acta Derm Venereol 2022; 102: adv00805.
- Ruggiero A, Fabbrocini G, Cinelli E, Ocampo Garza SS, Camela E, Megna M. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol 2022; 47: 561–567.
- Kolli SS, Gabros SD, Pona A, Cline A, Feldman SR. Tildrakizumab: a review of phase II and III clinical trials. Ann Pharmacother 2019; 53: 413–418.
- Reich K, Papp K, Blauvelt A, Tyring S, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. The Lancet 2017; 390: 276–288.
- Thaci D, Piaserico S, Warren RB, Gupta AK, Cantrell W, Draelos Z, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2). Br J Dermatol 2021; 185: 323–334.
- Reich K, Warren RB, Iversen L, Puig L, Pau-Charles I, Igarashi A, et al. Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br J Dermatol 2020; 182: 605–617.
- Langenbruch A, Radtke MA, Gutknecht M, Augustin M. Does the Dermatology Life Quality Index (DLQI) underestimate the disease-specific burden of psoriasis patients? J Eur Acad Dermatol Venereol 2019; 33: 123–127.
- Rencz F, Gulácsi L, Péntek M, Szegedi A, Remenyik É, Bata-Csörgő Z, et al. DLQI-R scoring improves the discriminatory power of the Dermatology Life Quality Index in patients with psoriasis, pemphigus and morphea. Br J Dermatol 2020; 182: 1167–1175.
- Rencz F, Gulácsi L, Péntek M, Poór A k., Sárdy M, Holló P, et al. Proposal of a new scoring formula for the Dermatology Life Quality Index in psoriasis. Br J Dermatol 2018; 179: 1102–1108.
- Bakirtzi K, Sotiriou E, Papadimitriou I, Sideris N, Vakirlis E, Lallas A, et al. Elderly patients with psoriasis: long-term efficacy and safety of modern treatments. J Dermatol Treat 2022; 33: 1339–1342.
- Gheno R, Cepparo JM, Rosca CE, Cotten A. Musculoskeletal disorders in the elderly. J Clin Imaging Sci 2012; 2: 39.
- Bechtold M, Palmer J, Valtos J, Iasiello C, Sowers J. Metabolic syndrome in the elderly. Curr Diab Rep 2006; 6: 64–71.
- Writing Groups Members, Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation 2010; 121: e46–e215.
- Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol 2018; 36: 21–28.
- Frieder J, Ryan C. Psoriasis and cardiovascular disorders. G Ital Dermatol Venereol 2016; 151: 678–693.
- Cuesta-Montero L, Belinchón I. Connective tissue diseases and psoriasis. Actas Dermosifiliogr 2011; 102: 487–497.
- European Medicines Agency (EMA). [Accessed 2022 Feb 10]. Available from: https://www.ema.europa.eu/en/documents/product-information/ilumetri-epar-product-information_en.pdf.
- Armstrong AW, Puig L, Joshi A, Skup M, Williams D, Li J, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol 2020; 156: 258–269.
- Barbieri JS, Shin DB, Syed MN, Takeshita J, Gelfand JM. Evaluation of the frequency of ‘not relevant’ responses on the dermatology life quality index by sociodemographic characteristics of patients with psoriasis. JAMA Dermatol 2020; 156: 446–450.
- Barbieri JS, Gelfand JM. Responsiveness of the EuroQol 5-dimension 3-level instrument, Dermatology Life Quality Index (DLQI) and DLQI-relevant for patients with psoriasis in the U.S.A. Br J Dermatol 2019; 181: 1088–1090.
- Rencz F, Poór AK, Péntek M, Holló P, Kárpáti S, Gulácsi L, et al. A detailed analysis of ‘not relevant’ responses on the DLQI in psoriasis: potential biases in treatment decisions. J Eur Acad Dermatol Venereol 2018; 32: 783–790.
- Barbieri JS, Gelfand JM. Influence of ‘not relevant’ responses on the Dermatology Life Quality Index (DLQI) for patients with psoriasis in the United States. JAMA Dermatol 2019; 155: 743–745.
- Rencz F, Gergely H, Wikonkal N, Gáspár K, Péntek M, Gulacsi L, et al. Dermatology Life Quality Index (DLQI) score bands are applicable to DLQI-Relevant (DLQI-R) scoring. J Eur Acad Dermatol Venereol 2020; 34: e484–e486.
- Rencz F, Gulácsi L, Péntek M, Szegedi A, Remenyik É, Bata-Csörgő Z, et al. DLQI-R scoring improves the discriminatory power of the Dermatology Life Quality Index in patients with psoriasis, pemphigus and morphea. Br J Dermatol 2020; 182: 1167–1175.
- van Lümig PPM, Driessen RJB, Berends M a. M, Boezeman JBM, van de Kerkhof PCM, de Jong EMGJ. Safety of treatment with biologics for psoriasis in daily practice: 5-year data. J Eur Acad Dermatol Venereol 2012; 26: 283–291.
- Howell ST, Cardwell LA, Feldman SR. Treating moderate-to-severe plaque psoriasis with guselkumab: a review of phase II and phase III trials. Ann Pharmacother 2018; 52: 380–387.
- Wan MT, Shin DB, Winthrop KL, Gelfand JM. The risk of respiratory tract infections and symptoms in psoriasis patients treated with interleukin 17 pathway–inhibiting biologics: A meta-estimate of pivotal trials relevant to decision making during the COVID-19 pandemic. J Am Acad Dermatol 2020; 83: 677–679.
- Rivera R, García-Doval I, Carretero G, Daudén E, Sánchez-Carazo J, Ferrándiz C, et al. BIOBADADERM, the Spanish Registry of adverse events associated with biologic drugs in dermatology: first report. Actas Dermosifiliogr 2011; 102: 132–141.
- Naldi L, Mercuri SR. Epidemiology of comorbidities in psoriasis. Dermatol Ther 2010; 23: 114–118.
- Singh RK, Lee KM, Ucmak D, Brodsky M, Atanelov Z, Farahnik B, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl) 2016; 6: 93–104.
- Yosipovitch G, Tang MBY. Practical management of psoriasis in the elderly: epidemiology, clinical aspects, quality of life, patient education and treatment options. Drugs Aging 2002; 19: 847–863.
- Drerup KA, Seemann C, Gerdes S, Mrowietz U. Effective and safe treatment of psoriatic disease with the anti-IL-23p19 biologic tildrakizumab: results of a real-world prospective cohort study in nonselected patients. Dermatology (Basel, Switzerland) 2022; 238: 615–619.
- Tsianakas A, Schwichtenberg U, Pierchalla P, Hinz T, Diemert S, Korge B. Real-world effectiveness and safety of tildrakizumab in long-term treatment of plaque psoriasis: Results from the non-interventional, prospective, multicentre study TILOT. J Eur Acad Dermatol Venereol 2023; 37: 85–92.