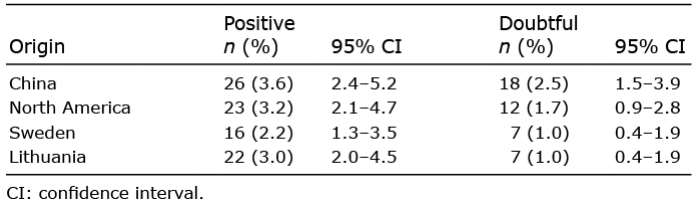
Table I. Positive patch-testing and doubtful reactions to propolis from 4 different origins among 722 patients
Department of Dermatology and Venereology, Region Västra Götaland, 1Sahlgrenska University Hospital, Gothenburg, 3Southern Älvsborg Hospital, Borås, 4Skaraborg Hospital, Skövde, 5Uddevalla Hospital, Uddevalla, and 2Department of Dermatology and Venereology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Propolis, which is produced by honeybees and is used in “natural” products, can cause contact allergy. The composition of propolis varies between regions, but little is known about how this variation affects contact allergenicity. The aims of this study were to investigate the frequency of propolis contact allergy in western Sweden, and whether the frequency varies according to the origin of the propolis. Patch-testing was performed using propolis from China, Lithuania, North America, and Sweden in 722 consecutive patients with dermatitis in western Sweden. Frequencies of positive patch-test reactions ranged from 2.4% to 3.6%. There were some, not statistically significant, differences in frequency of contact allergy to the 4 samples of propolis of different origins, with the highest frequency to the sample from China and the lowest frequency to the sample from Sweden. Concomitant positive patch-test reactions to plant and fragrance substances in the baseline series were common, most frequently to Myroxylon pereirae resin and colophonium.
Key words: propolis; allergic contact dermatitis; patch test; cross reactions.
Accepted Aug 13, 2020; Epub ahead of print Aug 19, 2020
Acta Derm Venereol 2020; 100: adv00256.
Corr: Gunnar Nyman, Department of Dermatology and Venereology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, SE-405 30 Gothenburg, Sweden. E-mail: nyman@cutis.nu
Propolis, which is produced by honeybees and used in so-called “natural” products, can cause contact allergy. The composition of propolis varies between regions. In this study Swedish patients with dermatitis were patch-tested for contact allergy to propolis originating from China, Lithuania, North America and Sweden. Propolis contact allergy was common, and there were only small differences in the frequency of contact allergy between the 4 samples of propolis of different origins. Concomitant positive patch-test reactions to plant and fragrance substances series were common. It is important to patch-test patients with dermatitis with propolis and to inform patients who have propolis contact allergy that they should avoid contact with it and with some plant and fragrance substances.
Propolis, or bee glue, is produced by honeybees. It is a lipophilic substance manufactured by bees from resinous exudates from plants around the hive, mixed with beeswax and β-glycosidase from the bee’s saliva (1). Propolis is used by bees for gluing and sealing cracks in the beehive and covering the area around the entrance to the hive. It has antimicrobial effects, which protect the colony against infections (1). Poplar buds (Populus spp) are the most important source of plant material for propolis in central Europe, but this may vary depending on the plants growing in the vicinity of the hive (1). In eastern parts of Russia birch buds (Betula spp) are a common source of propolis, and in South America rose-wood buds (Dahlbergia spp) and balsam apple buds (Clusia rosea L) are common sources (1). Propolis has been used by humans since ancient times, and is currently used in “natural” remedies and biocosmetics. It is often an impurity in beeswax (2, 3), and can appear in honey, intentionally or not. Beeswax and propolis are different products, although they are sometimes referred to as the same thing (4), and beeswax contains different amounts of propolis depending on how well refined or how chemically treated it is (5).
Propolis is a well-known allergen (2, 6–8), which sometimes causes occupational contact allergy (9–11). It is rather common as a contact allergen in many European countries, especially in eastern and middle Europe (3, 12). Frequencies of 0.5–17% of positive patch-test reactions have been recorded among patients with dermatitis (1, 3, 8). The current study is the first systematic investigation into the frequency of contact allergy to propolis among Swedish patients with dermatitis. There is a high number of concomitant patch-test reactions between propolis and beeswax (2, 13), and a concomitant reaction to honey is described (14). As a natural product propolis is not chemically defined, but more than 180 chemical substances have been detected in propolis, including benzyl benzoate, caffeates, flavonoids and cinnamic acid derivatives (15). The variation in composition makes it difficult to investigate contact allergy to propolis, and there has been little research into whether this variation affects the outcome of patch-testing. Propolis from different regions differs chemically (16) and in antimicrobial effect (17), but, to our knowledge, there has been only one study of the difference in patch-test reactions, using propolis from different locations in the British Isles (18).
Caffeic acid and its derivatives are thought to be the main haptens in propolis, but isoferulates, flavonoid aglycones and free aromatic acids may also be present (19, 20). Several of these substances are also known to occur in Myroxylon pereirae resin (MPR, Balsam of Peru) (21–23). There is a high number of concomitant reactions between propolis and fragrances or plant substances (21, 24, 25). Contact allergy to propolis is of importance for patients using different “natural remedies” and cosmetics containing propolis, honey or beeswax, since the latter may be contaminated with propolis (26). Beekeepers and people who manufacture products containing propolis are at risk of developing occupational allergic contact dermatitis to propolis (11, 18), as are musicians and string-instrument makers, since propolis is often a component of the varnish used on, for example, violins (7, 9).
The aims of this study were to investigate the frequency of contact allergy to propolis in patients with dermatitis in western Sweden, and to examine differences in the frequencies of contact allergy to propolis of different origins. A further aim was to study concomitant patch-test reactions between propolis, and fragrances and plant substances in the Swedish baseline series (https://ssdv.se/svenska-saellskapet-foer-arbets-och-miljoedermatologi-ssamd/utredning-av-hudallergi/svensk-basserie-2017).
Study population
A multicentre study was conducted at the 4 dermatology clinics in Västra Götaland Region in western Sweden, located in Borås, Gothenburg, Skövde and Uddevalla, during October 2016 to December 2017. Consecutive patients with dermatitis who were referred for patch-testing with the Swedish baseline series due to suspicion of allergic contact dermatitis were also tested with propolis of 4 different origins. A total of 722 patients were tested (511 females and 211 males: mean age 44.5 years; age range 18–86 years; females/males 71/29%).
Patch-test preparations
The patch-test preparations used in the Swedish baseline series were purchased from Chemotechnique Diagnostics (Vellinge, Sweden) by the respective clinics. The propolis preparations of Chinese and North American origin were purchased by the Gothenburg clinic from Chemotechnique Diagnostics and Smart Practice (Phoenix, AZ, USA), respectively. The propolis from the west coast of Sweden and Kaunas region of Lithuania were provided directly by a single beekeeper in each area and prepared by Chemotechnique Diagnostics (as 10% in petrolatum) in the same way as the propolis of Chinese origin in their usual range. All propolis preparations were distributed from Gothenburg to the participating clinics.
The study was approved by the local ethics committee (721-16).
Patch-testing
Patch-testing and reading of the patient’s results were carried out according to European Society of Contact Dermatitis (ESCD) guidelines (27). Finn chambers (8-mm diameter; Smart Practice) on Scanpor tape (Norgesplastr, Vennesla, Norway) were used in all centres, except Gothenburg, which used IQ Ultra chambers (8×8 mm; Chemotechnique Diagnostics). A 20 mg dose was applied with the Finn chamber and 25 mg with the IQ Ultra chamber. Relevance was assessed on the basis of patient history.
According to a previously presented scoring system for multicentre studies, the current study was of high quality (28).
Statistical analyses
All data were analysed using R version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Two-sample tests were performed using Wilcoxon rank sum test and Fisher’s exact test. For paired tests, the exact binomial test was used. All tests were 2-sided and p < 0.05 was considered statistically significant. Exact 95% confidence intervals (CI) of frequencies of contact allergy were calculated using OPENEPI (http://openepi.com). Venn diagrams were calculated using eulerAPE (29).
A total of 45 of the 722 patients in the study population had positive patch-test reactions to any of the types of propolis (6.2%; Table I). The most frequent reaction was to the propolis originating from China (3.6%) and the least frequent to the propolis from western Sweden (2.2%). There were small, but not statistically significant, differences in positive patch-test results between women and men.

Table I. Positive patch-testing and doubtful reactions to propolis from 4 different origins among 722 patients
Of the 45 patients with a positive patch-test to propolis, 23 (51%) had a reaction to only 1 of the 4 origins of propolis. A reaction only to propolis from China was found in 13 patients (29%), to propolis from North America only in 6 patients (13%) and to propolis from Sweden or Lithuania only, in 2 patients each (4%). Eight patients (18%) reacted to 2 and 3 types of propolis, respectively and 6 patients (13%) reacted to all 4 types of propolis. Evaluation of relevance was recorded in 33 (73%) of the patients who were patch-test positive to propolis. Current relevance was found in 9 (27%) of those recorded (20% of all propolis patch-test positive patients), earlier relevance was found in 2 patients (6%), and unclear relevance in 22 patients (67%).
Among the patients with positive patch-test to propolis of any of the 4 origins, the most frequent concomitant reactions to allergens in the baseline series were to MPR (43%), colophonium (23%) and Fragrance mix I (FMI) (16%) (Table II). The frequencies of concomitant reactions to these test preparations were also significantly higher than the frequencies of concomitant reactions among patients with negative patch-test reactions to propolis (Table II).
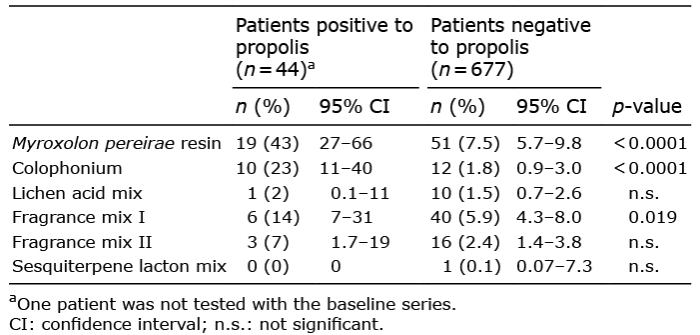
Table II. Concomitant positive patch-test reactions to plant and fragrance markers in the Swedish baseline series in patients positive to propolis of any origin and in patients negative to propolis
In total, there were 37 (5.1%) doubtful reactions to any type of propolis (Table I). The highest number of doubtful reactions was to the propolis from China, 18 (2.5%). Seven (1.0%) reactions, all at 2 of the centres, were considered irritant. Irritant reactions were seen mainly to the propolis originating from China and Sweden, each 3 reactions (0.4%).
Two late reactions were recorded: one patient (patient 1) reacted on day 24 after the application of patch-tests to propolis from China and North America and one patient reacted on day 11 (patient 2) after application of propolis from Sweden and Lithuania, and to MPR. When retested 3 months (patient 1) and 5 months (patient 2) later, both patients had positive patch-test reactions on day 3 to the same types of propolis, and patient 2 to MPR also.
This study found a high frequency of positive patch-test reactions to propolis originating in China, Lithuania, North America and Sweden (6.2%), in the upper range of the frequencies that have been reported previously (1, 8).
When comparing contact allergy to propolis among beekeepers in the British Isles, 11 out of 13 (84%) were found to react to propolis from other locations in the British Isles in addition to reacting to their own propolis (18). The different types of propolis in the present study originate from regions much further apart geographically, which may result in greater differences in composition, and explain why fewer concomitant reactions were found. Patients patch-testing positive to propolis originating from China (n = 26) had concomitant positive reactions to propolis from Sweden in 8 cases (31%) and patients positive to propolis from Sweden (n = 16) had concomitant positive reactions to propolis from China in 8 cases (50%). This probably reflects greater differences in composition of propolis from different parts of the world compared with propolis from different parts of the British Isles.
The current study could not demonstrate any statistically significant differences between the propolis from 4 different regions of the world, or between the sexes. In females, positive patch-test reactions to propolis from China were most frequent (4.1%) and, in men, positive reactions to propolis from Sweden were most frequent (3.3%), but those differences were also not significant.
It is difficult to determine the origin of propolis in different products, as the substances may be transported across several countries before a product is manufactured. One explanation for the difference in frequency between positive patch-test reactions to propolis originating from China and Sweden among Swedish patients with dermatitis may be that propolis in consumer products is purchased from the same sources used by the manufacturers of the patch-test material. The results may have been different if the study had tested Swedish beekeepers, who are likely to be more exposed to propolis from their own bees. Some of the patients with a positive patch-test to propolis with unclear relevance might, in fact, have a current relevance, as there is a high frequency of concomitant reactions between propolis and beeswax (14), and exposure to the latter has not always been recorded.
In routine testing it does not seem reasonable to use several types of propolis, but using the propolis originating from China will discover most positive reactions. Furthermore, it detects the highest number of positive patch-test reactions (3.7%) as well as the highest number of unique reactions. This frequency of positive reactions is within the range found previously in other European countries (1).
There are many doubtful reactions, which may be a limitation when testing propolis in regular screening. This has been reported in previous studies of patch-testing with propolis (8). In general, a high frequency of doubtful reactions could be due to the test concentration being either too high (30) or too low (31–33). We chose a test concentration of 10%, as this is the recommended concentration used most commonly, although there are some investigators using 5% and 20% (3, 34). Since commercially available propolis test preparations from China and North America have a concentration of 10%, this concentration was also used for the other 2 test preparations from Sweden and Lithuania to enable comparison of the results.
According to ESCD patch-test guidelines (27), the 2 late reactions are to be considered proven as active sensitizations. It has, however, been demonstrated that such late reactions can nevertheless appear in previously sensitized individuals, as there are indices pointing to late reactions being a sign of too low patch-test concentrations (35–37). To our knowledge, there are no previous reports of active sensitization to propolis, even though propolis has been used for patch-testing for many years, including in higher concentrations. It is, however, of utmost importance to be alert for late reactions in the future. Further research is needed to determine the appropriate test concentrations of propolis.
Although concomitant reactions were expected between propolis and plant and fragrance substances in the Swedish baseline series, the high frequencies of concomitant patch-test reactions found between propolis and MPR, colophonium and FM I are noteworthy (Table II, Fig. 1). Plant-derived products and fragrances with a natural origin that are used for patch-testing may share several components with propolis (e.g. cinnamal, cinnamyl alcohol, cinnamic acid and benzyl alcohol) (1). This may cause concomitant reactivity. There is also the possibility of co-sensitization, as natural cosmetics often contain many plant extracts in addition to propolis or beeswax. Patients with positive patch-testing to MPR, colophonium or FM I, should be warned about possible contact allergy to propolis.
The current study has some limitations. Information about the plants growing near the beehives at the time the bees are collecting material for making propolis is lacking. As in most multicentre studies, patch-test readings were performed by different colleagues in different clinics. For practical reasons, these limitations are difficult to overcome, but, in the first case, it may be possible instead to investigate the presence of haptens in different types of propolis.
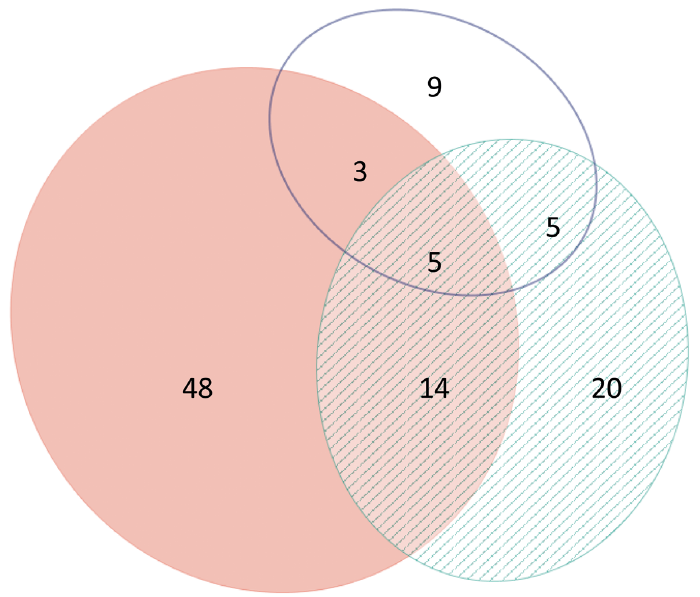
Fig. 1. Venn diagram showing number of patients patch-testing positive to Myroxolon pereire resin (MPR), pink (n = 70), colophonium, white (n = 22) and propolis, dashed green, (n = 44), and concomitant reactions between them. Concomitant positive patch-test reactions between MPR and colophonium: 11; MPR and propolis: 19; colophonium and propolis: 10, and between all test substances: 5.
The study was supported by a grant from the Local Research & Development Council of Södra Älvsborg.
The authors thank Martin Gillstedt for statistical analysis, Britt-Marie Ehn for patch-testing and study logistics. We thank the apiary “Djäknegårdens honung” in County Halland, and the beekeeper Antanas Kliu?inskas in Kaunas region, for providing raw propolis from Sweden and Lithuania, respectively. Thanks are also due to colleague Aist? Beliauskien? for transporting the Lithuanian propolis to us.
The authors have no conflicts of interest to declare.