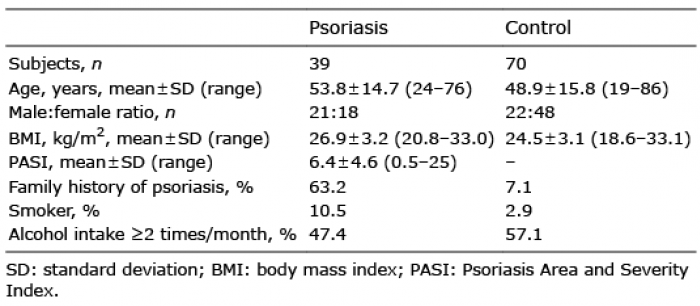
Table I. Patients’ demographic and clinical characteristics
1Division of Dermatology and Venereology, Region Jönköping County, 2Division of Medical Diagnostics, Region Jönköping County, Jönköping, and 3Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden
Studies have shown differences in the skin and gut bacterial microbiomes in patients with psoriasis, but the pharyngeal microbiome has not been studied previously. The aim of this study was to investigate differ-ences in the bacterial microbiome of the pharynx and skin of patients with psoriasis compared with healthy controls. Swabs were taken from the pharynx and elbow skin of 39 patients with psoriasis and 70 controls. Microbiomes were characterized by sequencing 16S rRNA genes on the Illumina MiSeq platform. Significant differences were found in alpha and beta diversity in the skin, but not in the pharynx. Significant differences were also found between several phyla and genera in both skin and pharynx. The severity of psoriasis did not correlate with any genera in the pharynx, but with Capnocytophaga, Leptotrichia, Abiotrophia and Tannerella in the skin. The composition of the pharyn-geal and skin microbiome may be of importance in the patho-genesis of psoriasis.
Key words: microbiome; psoriasis; pharynx; skin.
Accepted Aug 21, 2020; Epub ahead of print Aug 26, 2020
Acta Derm Venereol 2020; 100: adv00273.
doi: 10.2340/00015555-3619
Corr: Malin Assarsson, Division of Dermatology, Ryhov Hospital, SE-551 85 Jönköping, Sweden. E-mail: malin.assarsson@rjl.se
Psoriasis is thought to be linked to dysbiosis of the micro-biome of the gut and skin. This study analysed samples from the pharynx and elbow skin of 39 patients with psoriasis and 70 controls and found differences in both alpha and beta diversity. The severity of psoriasis did not correlate with any specific bacteria in the pharynx, but did correlate with several types of bacteria in the skin. The composition of the pharyngeal and skin microbiome may be of importance in the pathogenesis of psoriasis.
Psoriasis is a common immune-mediated inflammatory disease with an estimated prevalence of 2–3% (1). It is associated with a significantly increased risk of comorbidities, such as myocardial infarction, inflammatory bowel disease (IBD) and obesity (2, 3). It is well-known that bacterial colonization of various parts of the body can both trigger and exacerbate psoriasis (4, 5), and the microbiome, of the gut, in particular, has been implicated in a variety of other inflammatory and systemic autoimmune diseases, such as diabetes mellitus, rheumatoid arthritis and IBD (6–8). It has been proposed that psoriasis is caused by a breakdown of immune tolerance to the microbiome of the skin or gut, in which bacteria in genetically predisposed individuals activate the innate immune system, inducing an adaptive immune response (9). Stehlikova et al. used an experimental mouse model of psoriasis induced by imiquimod to suggest that the intestinal and skin microbiome directly regulates skin inflammation, emphasizing the importance of the microbiome in the pathogenesis of psoriasis (10).
Several studies have attempted to characterize the skin microbiome in psoriasis plaques, with inconsistent results. Some studies found the microbiome of psoriasis plaques to be more diverse than that of unaffected skin sites or healthy controls (11, 12), while others saw a trend towards decreased diversity of psoriasis lesions (13, 14). Our group showed that treatment of psoriasis with narrowband ultraviolet B (nUVB) affects the microbiome (15). The results of these studies might indicate that skin bacterial communities in psoriasis may be shifted in a modest, but significant, manner, but, to date, no specific disease-associated microbial signature of psoriasis has been found. However, it cannot be excluded that the differences between studies could be due to different sampling and analysis methods.
The microbiome of the gut has also been associated with a variety of inflammatory and systemic autoimmune diseases, including psoriasis (16). As for studies of the skin microbiome, studies of the gut microbiome in psoriasis show inconsistent results. One study shows a more diverse gut microbiome in patients with psoriasis compared with controls (17), while others show a less diverse gut microbiome (18, 19). Another study found no significant differences in diversity, but found a higher abundance of Bacteroidia and a lower abundance of Firmicutes in psoriatic patients compared with healthy controls (20). This is in agreement with an earlier study (21). Yet another study found a decreased abundance of Akkermansia muciniphila in psoriatic patients, which is interesting, since Akkermansia muciniphila is thought to have a function in the pathogenesis of IBD and obesity (22).
The most well-known bacterial trigger of psoriasis is Streptococcal infections of the upper respiratory tract, which is known to induce mainly guttate psoriasis (23–26). Tonsils from patients with psoriasis have been shown to have a higher frequency of cutaneous lymphocyte-associated antigen (CLA) CD4+ and CD8+ T-cells, and a higher frequency of tonsil T-cells expressing the IL-23 receptor, suggesting a dysregulated immune response in the tonsils of psoriatic patients (27). Ruiz-Romeo et al. found that Streptococcus pyogenes extracts induce a preferential Th17 response in patients with psoriasis (28), suggesting a direct involvement of Streptococcal infection in the pathogenesis of psoriasis. However, according to a recent Cochrane Review (29), the link between psoriasis and Streptococcal infection is suspected but not proven. Despite the suspected link between bacterial colonization in the pharynx and psoriasis, the pharyngeal bacterial microbiome has not previously been studied in patients with psoriasis.
The aim of this study is to investigate the differences in the bacterial microbiome of the pharynx and skin in patients with psoriasis and healthy controls.
Study subjects
A total of 50 patients with plaque-type psoriasis and 77 healthy controls were enrolled in this study. For 6 control cases PCR ampli-fication failed, for 3 patients with psoriasis the DNA-purification protocol yielded insufficient quantities of DNA to permit analysis, and for 8 patients with psoriasis the sequencing generated too few reads, excluding these individuals from the study. After inclusion in the study one control patient was found to have inflammatory bowel disease and was therefore also excluded. Finally, 39 patients with psoriasis and 70 controls were included in the data analysis. None of the patients had used topical antiseptics, oral antibiotics, systemic anti-inflammatory or immune-modulating treatment 3 months prior to entering the study. The patients with psoriasis had not used topical corticosteroids on the target lesion 2 weeks prior to entering the study. All included participants had a good oral health status with no known diseases of the oral cavity. Exclusion criteria were: pregnancy, tanning or intensive sun exposure 2 weeks prior to enrolling in the study, age under 18 years, known malignancy, psoriatic arthritis or other systemic inflammatory condition, as well as symptoms of infection at the time of sample collection. All included participants lived in the same geographical are of Sweden, which decreases the impact of diet and the environment. All subjects gave written informed consent according to protocols approved by the ethics committee, Linköping University, Linköping, Sweden (approval number 2014/179-31). Sex, age, height, weight, current diseases and medications, smoking and alcohol habits, and family history of psoriasis were recorded. For patients with psoriasis, current psoriasis treatment was recorded and an assessment of disease severity using the Psoriasis Area and Severity Index (PASI) was calculated by a trained dermatology nurse. The same nurse carried out all assessments. Seven patients had mild psoriasis (PASI<3), 24 had moderate psoriasis (PASI 3–9) and 8 had severe psoriasis (PASI≥10). Patients’ demographic and clinical characteristics are summarized in Table I. The participants other diseases and medications are shown in Table SI.

Table I. Patients’ demographic and clinical characteristics
Sample collection
A sample was taken from the pharynx, lesional skin of the elbow and non-lesional skin at an adjacent cutaneous location at least 10 cm from lesional skin in patients with psoriasis, and from the pharynx and elbow skin in healthy controls. The samples were taken from the pharynx by swabbing both tonsils and from the skin by swabbing a 4 × 4 cm area with a flocked swab soaked in 1 ml liquid Amies (ESwab™, Copan Diagnostics Inc., Murrieta, CA, USA). Since the colonization of bacteria in the skin is dependent on skin site (30), all samples were taken from the elbow area, or, for non-lesional skin samples in psoriatic patients, from skin adjacent to the elbow area. The samples were stored at –20°C until DNA isolation.
DNA extraction and sequencing
The entire liquid sample suspension from the ESwab was transferred into 1.5-ml reaction tubes and centrifuged at 16,000×g. The pellet was suspended in 180 µl G2 buffer (Qiagen, Hilden, Germany) and 20 µl Proteinase K (Qiagen) and incubated for 30 min at 56°C and then 5 min at 98°C. DNA extraction was performed in a Biorobot EZ1 (Qiagen) with the EZ1 Tissue kit v.2.0 (Qiagen) according to the manufacturer’s protocol. The sample volume for purification was 200 µl, and the purified DNA was eluted in 100 µl. A negative template control, 1 centrifuged E-swab, was prepared identically to the samples. The DNA samples were stored at –20°C. Samples were prepared for 16S rDNA sequencing according to the 16S Metagenomic Sequencing Library Preparation guide part #15044223 Rev. B. on a MiSeq instrument (Illumina, CA, USA). The protocol describes the steps to amplify the V3 and V4 region using a limited cycle PCR, to add Illumina sequencing adapters and dual-index barcodes to the amplicon target. Quality control was assessed using a DNA 1000 chip (Agilent Technologies, CA, USA) run in a Bioanalyzer 2100 (Agilent Technologies). DNA concentration was measured on a Qubit instrument with dsDNA HS Assay kit (Invitrogen, CA, USA). The only difference from the Illumina protocol was that the first PCR was duplicated and the same samples were then pooled before the first PCR clean up, and that the negative template control was controlled after the first PCR on a Bioanalyzer DNA 1000 chip. All negative template controls were negative after the first PCR and therefore not further processed. Finally, 35% Phix Control and 6 pM amplicon library were used. Quality of sequencing was controlled by FastQC Java script (http://www.bioinformatics.babraham.ac.uk/projects/fastqc).
Sequence processing, classification, and data analysis
FASTQ files were imported as paired-end reads into CLC Genomic Workbench (http://www.clcbio.com Version 20.0 Qiagen, Hilden, Germany) for processing and analysis. Sequences were trimmed for quality (score 0.05, 0 ambiguities). Overlapping pairs were merged into a single sequence and merged sequences were further trimmed to remove PCR-primers, only keeping sequences ≥ 200–≤ 350. Samples were filtered based on number of reads with a minimum of 40,000 reads. A reference-based approach (SILVA, 16S v. 132, Max Planck Institute for Marine Microbiology and Jacobs University, Bremen, Germany) was used for clustering, with a similarity threshold of 97%, and chimeric sequences were removed. The number of reads in operational taxonomic units (OTUs) varied from 41,479 to 598,192 in the pharynx with a median number of reads of 151,731. In the skin, the number of reads in OTUs varied from 28,756 to 376,310, with a median number of reads of 140,173. The top 150 most abundant OTUs were aligned using MUSCLE (31) with default settings and a maximum likelihood-based phylogenetic tree was generated using neighbour joining and Jukes-Cantor nucleotide substitution model. Read counts were down sampled (rarefied) to the lowest count (50,413) across all samples for measures of alpha and beta diversity. Measures of alpha diversity using Shannon diversity index were calculated with a sample depth of 8,077 and visualized using alpha diversity rarefaction curves (20 points, no replacement, and 100 replicated at each point). Mann–Whitney U test was used for statistical analysis of alpha diversity in the pharynx and Kruskal–Wallis analysis of variance (ANOVA) in the skin, using the Statistica software version 13.5.0.17, TIBCO Software, Palo Alto,, CA, USA. Correlation between PASI and Shannon index was analysed using Spearman’s rank order correlation, also in Statistica software. Beta diversity was calculated using unweighted UniFrac distance, and significance testing was performed using the permutational multivariate analysis of variance (PERMANOVA) test in the CLC software. Each of the 3 first principal co-ordinates analysis (PCoA) axes was significance tested for group differences (e.g. pharynx from control samples vs pharynx samples from patients with psoriasis) using the Wilcoxon rank sum test (wilcox.test() function, R version 3.6.0), and adjusted for multiple testing using the p.adjust() function and a FDR p-value of 0.05. For each OTU used as input for the PCoA a correlation was calculated between count data and coordinates of the 3 first PCoA-axis using the corr.test() function of the R package psych version 1.9.12.3. For all significant correlations (FDR-adjusted p-value of 0.05) trans-formed OTU count data were investigated for group differences in the same manner as described above. The transformed OTU count data was obtained by scaling using the trimmed mean of M-values and the calcNormFactor()-function from the EdgeR package version 3.28.0, and then log2-transformed using the normalized library sizes and the cpm()-function from EdgeR. Correlation between beta diversity and PASI was calculated using the mean unweighted UniFrac distance between each psoriasis patient and all the controls, and analysed with Spearman’s rank order correlation in the Statistica software.
The highly abundant taxa (total abundance > 0.1% of total number of OTUs) were pairwise compared with regard to sample type at phylum and genus level using a generalized linear model assuming read counts that follow a negative binomial distribution, and with a dispersion corrections similar to the EdgeR method (32) in the CLC software. Significance testing was conducted using a false discovery rate (FDR) corrected p-value of 0.05.
To analyse correlations between the pharyngeal and skin microbiome and between disease severity and bacterial abundance, the count data and PASI scores were imported into the R statistical environment version 3.6.0. The count data was scaled using trimmed mean of M-values and the calcNormFactor()-function from the EdgeR package version 3.28.0, and then log2-transformed using the normalized library sizes and the cpm()-function from EdgeR. 1278 species and 695 genera were present in both skin and pharyngeal samples. The genera and species present in both skin and pharyngeal samples were filtered leaving only taxonomic groups where less than 50% of samples had zero counts. Correlation was analysed using Spearman’s rank order correlation with the cor.test()-function. Significance testing was conducted using the padjust()-function and a FDR corrected p-value of 0.05.
Significant group differences in species richness and diversity
In the pharynx, there were no significance differences in alpha diversity using Shannon diversity index when comparing patients with psoriasis with controls (Fig. 1a, p > 0.05). In the skin, lesional skin in patients with psoriasis had significantly higher alpha diversity than non-lesional skin (p < 0.001) and controls (p < 0.01) (Fig. 1b).
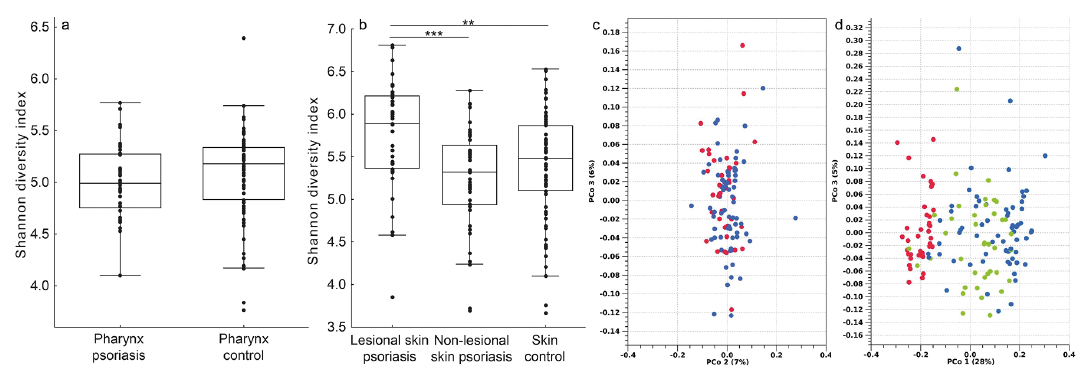
Fig. 1. (a) No significant difference in the alpha diversity of the pharyngeal microbiome between healthy controls and patients with psoriasis. (Line=median, box=first and third quartile, whiskers=non-outlier range). (b) Significant differences in the alpha diversity in the skin microbiome when comparing healthy controls, lesional and non-lesional skin in patients with psoriasis. (**p < 0.01, ***p < 0.001 , line=median, box=first and third quartile, whiskers=non-outlier range). (c) Principal coordinate analysis (PCoA) of beta diversity using unweighted UniFrac showing no significant differences between the pharynx of healthy controls (blue) and patients with psoriasis (red); p > 0.05). (d) PCoA of beta diversity using Unweighted UniFrac with significant differences between skin in healthy controls (blue) and lesional skin in patients with psoriasis (red) (p < 0.001), between skin in healthy controls and non-lesional skin patients with psoriasis (green) (p < 0.001), and between lesional and non-lesional skin of patients with psoriasis (p < 0.001).
In the pharynx, there was no difference in beta diversity between patients with psoriasis and controls (Fig. 1c, p > 0.05). In the skin, there were significant differences in beta diversity between controls and lesional skin in patients with psoriasis (p < 0.001), between controls and non-lesional skin in patients with psoriasis (p < 0.01), and between lesional and non-lesional skin in patients with psoriasis (p < 0.001). Fig. 1d shows a PCoA of the beta diversity of the skin. In the skin, PC1 is the main axis separating lesional, non-lesional and control skin, and the OTUs that correlated with the first PCoA-axis were ambiguous taxa of the genera Corynebacterium, Peptostreptococcus, Cutibacterium, Lawsonella, Acidovorax, Tepidiomonas, Brevundimonas and Sphingomonas.
No correlation between severity of psoriasis and bacterial diversity
The data in the current study showed no correlation be-tween PASI and alpha diversity using Shannon diversity index in the pharynx of patients with psoriasis (Fig. S1A, Spearman’s R = –0.06, p > 0.05), in lesional skin (Fig. S1B, Spearman’s R = 0.10, p > 0.05), or in non-lesional skin (Spearman’s R = 0.04, p > 0.05). No correlation was found between beta diversity and PASI in the pharynx (Fig. S2A, Spearman’s R = 0.17, p > 0.05) or lesional skin (Fig. S2B, Spearman’s R = –0.13, p > 0.05) of patients with psoriasis.
Distribution of the bacterial microbiome in the pharynx
The majority of bacteria in the pharynx fall into 2 dominating phyla, for both patients with psoriasis and controls; Bacteroidetes and Firmicutes (Fig. 2a). The relative abundance of the phylum Actinobacteria were significantly lower in patients with psoriasis compared with controls (Fig. 2b).
Prevotella 7, Veillonella and Haemophilus were the 3 most common genera of the pharynx, both in patients with psoriasis and in controls (Fig. 2c). The relative abundance of the genus Prevotella was significantly higher in the pharynx of patients with psoriasis compared with controls, while the genera Campylobacter, Cutibacterium, Tepidimonas, Acidovorax, Staphylococcus and ambiguous taxa of Absconditabacteriales order were significantly lower in patients with psoriasis compared with controls (Fig. 2d).
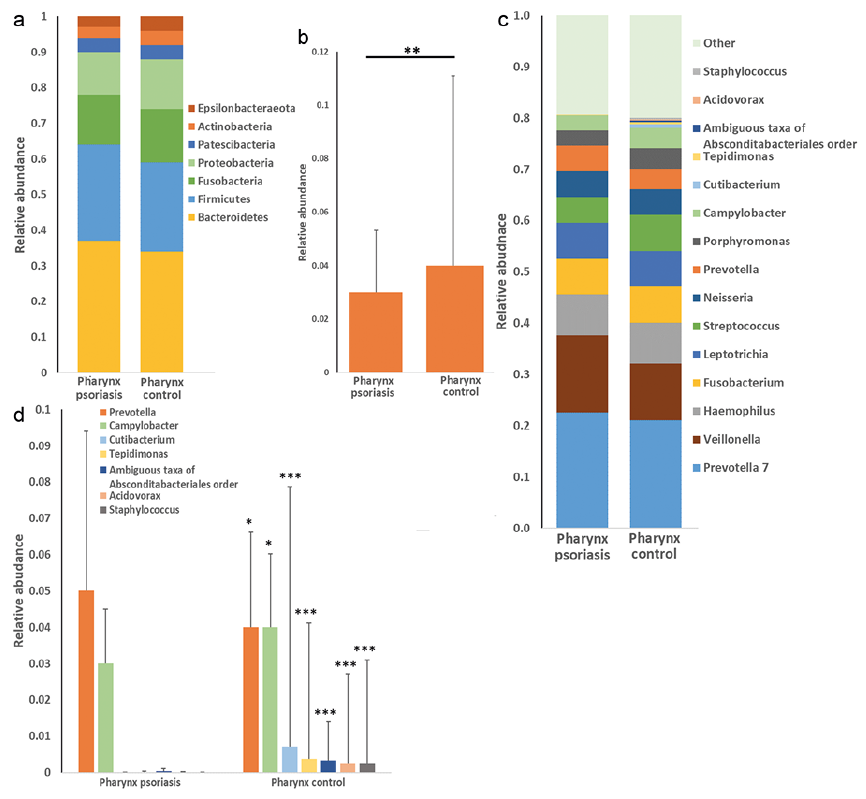
Fig. 2. (a) Bar chart showing relative abundance of bacteria in the pharynx at the phylum level in patients with psoriasis and controls. (b) Significant differences in the pharynx shown for the phylum Actinobacteria (false discovery rate (FDR); **p < 0.01). (c) Bar chart showing relative abundance of bacteria in the pharynx at the genus level in patients with psoriasis and controls. (d) Significant differences in the pharynx shown for the genera Prevotella, Campylobacter, Cutibacterium, Tepidimonas, ambiguous taxa of Absconditabacteriales order, Acidovorax and Staphylococcus. (FDR; *p < 0.05, ***p < 0.001).
Distribution of the bacterial microbiome of the skin
The 3 most common phyla of the skin both in patients with psoriasis and in controls were Firmicutes, Actinobacteria and Proteobacteria (Fig. S3a). There were no significant differences between the phyla of lesional and non-lesional skin in patients with psoriasis. Lesional skin of patients with psoriasis had significantly higher relative abundance of the phyla Firmicutes and Protebacteria and significantly lower relative abundance of Fusobacteria, and Cyanobacteria compared with controls (Fig. S3b).
The 3 most common genera of the skin were in lesional skin of patients with psoriasis Staphylococcus, Tepidimonas and Corynebacterium 1, in non-lesional skin of patients with psoriasis Staphylococcus, Cutibacterium and Tepidimonas, and in controls Staphylococcus, Cutibacterium and Corynebacterium 1 (Fig. S3c). Significant differences in the distribution of genera are shown in Table II.
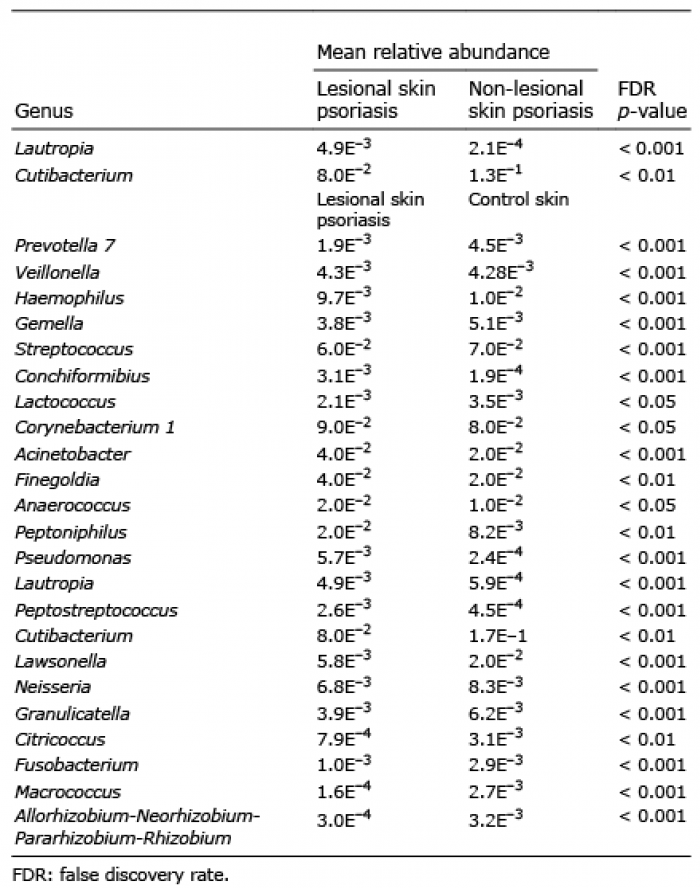
Table II. Significant differences in relative abundance on genus level in lesional and non-lesional skin in patients with psoriasis (n=39) and in lesional skin of patients with psoriasis and healthy skin in control patients (n = 70)
Correlation between the bacterial microbiome in the pharynx and in the skin
There was no correlation between the alpha diversity, measured using Shannon diversity index, in lesional skin compared with in the pharynx in patients with psoriasis (Fig. S4A, Spearman’s R = –0.05, p > 0.05) or between healthy skin and pharynx in control patients (Fig. S4B, Spearman’s R = 0.09, p > 0.05).
In both patients with psoriasis and in controls, there was a significant correlation between the skin and pharyngeal abundance for several different genera and species (Table III). The strongest correlations were in patients with psoriasis Rothia mucilaginosa and uncultured bacterium of the Actinobacillus and Parvimonas genus. In control patients, the strongest correlations were for uncultured bacterium of the Tepidimonas, Acidovorax, and Rothia genus. Streptococcus gordonii correlated only in patients with psoriasis and Streptococcus salivarius only in controls. An uncultured bacterium of the Fusobacterium genus correlated in patients with psoriasis, but not in controls.
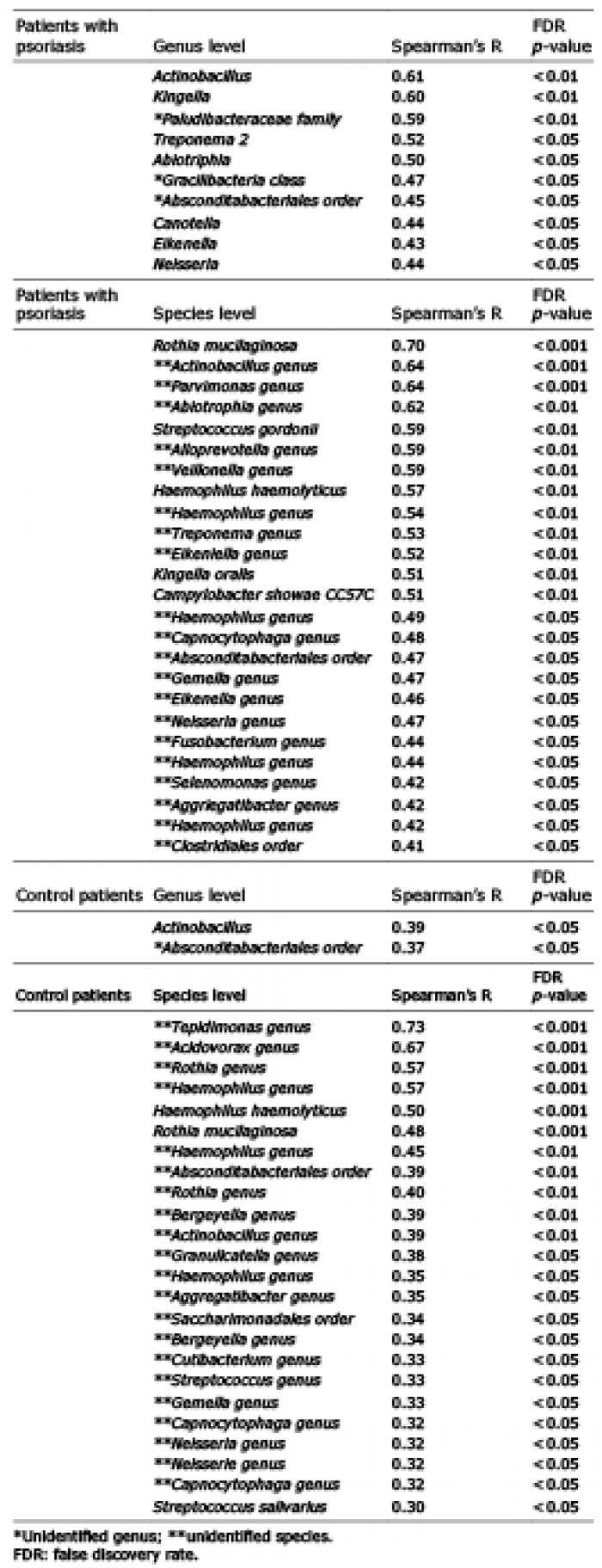
Table III. Significant correlations between pharyngeal and skin abundance at the genus and species level in patients with psoriasis (n=39) and control patients (n=70)
Correlation between disease severity and bacterial abundance
In the pharynx of patients with psoriasis, there were no significant correlations between bacterial abundance and PASI on either genus or species level, when p-values were adjusted for multiple testing.
In the skin of patients with psoriasis, there was a significant correlation between bacterial abundance and PASI for the genera Capnocytophaga (Spearman’s R = 0.54, FDR p < 0.05), Leptotrichia (Spearman’s R = 0.54, FDR p < 0.05), Abiotrophia (Spearman’s R = 0.51, FDR p < 0.05), and Tannerella (Spearman’s R = 0.51, FDR p < 0.05). On the species level, there was a significant correlation for ambiguous taxa of the Leptotrichia genus (Spearman’s R = 0.55, FDR p < 0.05) and uncultured bacterium of the Capnocytophaga genus (Spearman’s R = 0.53, FDR p < 0.05) and the Abiotrophia genus (Spearman’s R = 0.53, FDR p < 0.05).
This study analyses, for the first time, differences in the pharyngeal bacterial microbiome between patients with psoriasis and controls.
No significant difference was found in the relative abundance of the genus Streptococcus in the pharynx of psoriatic patients compared with controls. Also, no correlation was found between the severity of psoriasis and the relative abundance of Streptococcus. In the skin, the relative abundance of Streptococcus was significantly lower in lesional skin of patients with psoriasis compared with controls. It is possible, since Streptococcus is such a diverse genus, that its role could be better understood at the species level than at genus level. A correlation was found between the abundance of Streptococcus gordonii in the pharynx and skin in psoriasis patients and Streptococcus salivarius in controls. A previous study has reported sore throat, without confirmed streptococcal infections, as an aggravating factor for psoriasis, suggesting that subclinical infections and other, so far unidentified, pathogens in the pharynx also contribute to psoriasis exacerbations (33). None of the patients with psoriasis included in the current study had any symptoms of sore throat when the samples were taken. This might explain why no significant differences were found in the abundance of Streptococcus in the pharynx and no correlation with disease severity. If patients with symptoms of a throat infection had been included, the results might have been different.
No significant differences in alpha or beta diversity were found in the microbiome of the pharynx in patients with psoriasis and controls, but significantly higher alpha diversity was found in lesional skin of patients with psoriasis, and significant differences were also found in beta diversity between lesional skin and healthy skin of control patients. Among the OTUs that correlated with the PCO axes are OTUs in the genera Corynebacterium and Cutibacterium, where Corynebacterium was significantly higher and Cutibacterium significantly lower in lesional skin of psoriasis patients. A previous study has identified an imbalance between Corynebacterium and Cutibacterium in the lesional skin of psoriasis, with a higher proportion of Corynebacterium and lower proportion of Cutibacterium (34). A reduced level of Cutibacterium in lesional skin has also been found in 2 other studies (11, 35). The authors of the first study speculate that the dry environment of psoriatic lesions might not be suitable for Cutibacterium, which prefers a moist, sebaceous skin, while Corynebacterium is sensitive to low pH and is therefore less common in sebaceous skin (36), which also seems to be true for the current study.
Other chronic inflammatory diseases similar to psoriasis, such as Crohn’s disease (CD), show differences in the microbiome of the gut similar to the differences found in the skin in the current study. The relative abundance of the genus Prevotella was significantly lower in the skin of patients with psoriasis in the current study and has also been shown to be significantly decreased in the bowels of patients with CD (37). In the pharynx of the psoriasis patients in the current study, however, the relative abundance of Prevotella was significantly increased. The relative abundance of Pseudomonas was significantly higher in the skin of patients with psoriasis in the current study and has also been shown to be significantly higher in the ileum of children with CD (38).
The results of the current study showed no significant correlation between the severity of psoriasis and the bacterial abundance in the pharynx. This could indicate that it is not the level of inflammation that leads to changes in the microbiome of the pharynx, but perhaps instead the genetic differences between patients with psoriasis and controls. In the skin, 4 genera correlated with severity: Capnocytophaga, Leptotrichia, Abiotrophia and Tannerella. These genera on the skin have, to our knowledge, not previously been associated with psoriasis or other inflammatory diseases, and no significant difference was found in the relative abundance of these genera when comparing the skin of patients with psoriasis with that of healthy controls. The results of the current study suggest that these genera could influence the severity of psoriasis, but more studies are needed to determine the significance of these results.
When comparing the bacterial abundance of the pharynx with that of the skin, patients with psoriasis and controls had a similar number of species that correlated. Veillonella correlated only in patients with psoriasis, not controls in the current study. The abundance of Veillonella has previously been shown to correlate with inflammatory markers in psoriasis patients, indicating that Veillonella might play a pro-inflammatory role in the pathogenesis of psoriasis. Knox et al. (39) compared the gut microbiome of several immune-mediated inflammatory diseases, including IBD, multiple sclerosis and rheumatoid arthritis. From these results they made a list of candidate microorganisms considered highly interesting in the context of immune-mediated inflammatory diseases, including, among others, Akkermansia, Ruminococcus, Turucibacter, Lactobacillus, Collinsella and Eggerthella (39). In the current study no significant differences were found in these genera when comparing either pharynx or skin of psoriasis patients with controls, and no correlation was found between the relative abundance of these genera and the severity of psoriasis, indicating that they are not involved in the pathogenesis of psoriasis.
A limitation of the present study is that the patients and controls were not matched when included in the study, leading to differences between patients with psoriasis and controls regarding age and sex distribution. Many factors can influence the results of microbiome analysis. The current study excluded patients and controls with known systemic inflammatory conditions, but other health conditions and medications were allowed. It cannot be excluded that differences in medications and other known or unknown health conditions influence the results of microbiome studies. Patients with psoriasis had a significantly higher body mass index (BMI) and a lower percentage of regular alcohol intake compared with controls. The mean BMI in the current study in patients with psoriasis was 26.9 kg/m2 and in controls 24.5 kg/m2. Several studies have shown differences in the gut microbiome in patients with obesity (40–42). To our knowledge, there are no studies of the pharyngeal microbiome with regards to obesity, but it cannot be excluded that not only the gut microbiome, but also the pharyngeal microbiome is influenced by obesity and that this has influenced the results of the current study. Four patients with psoriasis and 2 healthy controls were smokers. Smoking has previously been shown in several studies to affect the microbiome of the oral cavity and the gut (43–45). It cannot be excluded that smoking influenced the results of the current study, but since the number of smokers was so low, it is not possible to draw any conclusions. Pharyngeal samples were taken from the patients and controls without regard to the last time they ate, drank, brushed teeth or smoked, and it also cannot be excluded that this has influenced the current results. None of our patients received oral antibiotics, anti-inflammatory treatment or immune-modulating treatment in the last 3 months prior to inclusion in the study. However, studies show that the microbiome of the gut can be affected long term after the intake of antibi-otics (46), and it cannot be excluded that the microbiome of the pharynx is also influenced for a longer time than 3 months, which makes the results of the current study less certain.
In conclusion, this study found significant differences in the microbiome of the pharynx and in the skin between psoriatic patients and healthy controls, which could be of importance in the pathogenesis of psoriasis.
This study was supported by grants from the Swedish Psoriasis Foundation and from Futurum, The Academy of Healthcare, Region Jönköping County, Sweden. The authors are grateful for the help of Marita Skarstedt, Division of Medical Diagnostics and Siv Nilsson, Division of Dermatology, Region Jönköping County, Sweden.
The authors have no conflicts of interest to declare.