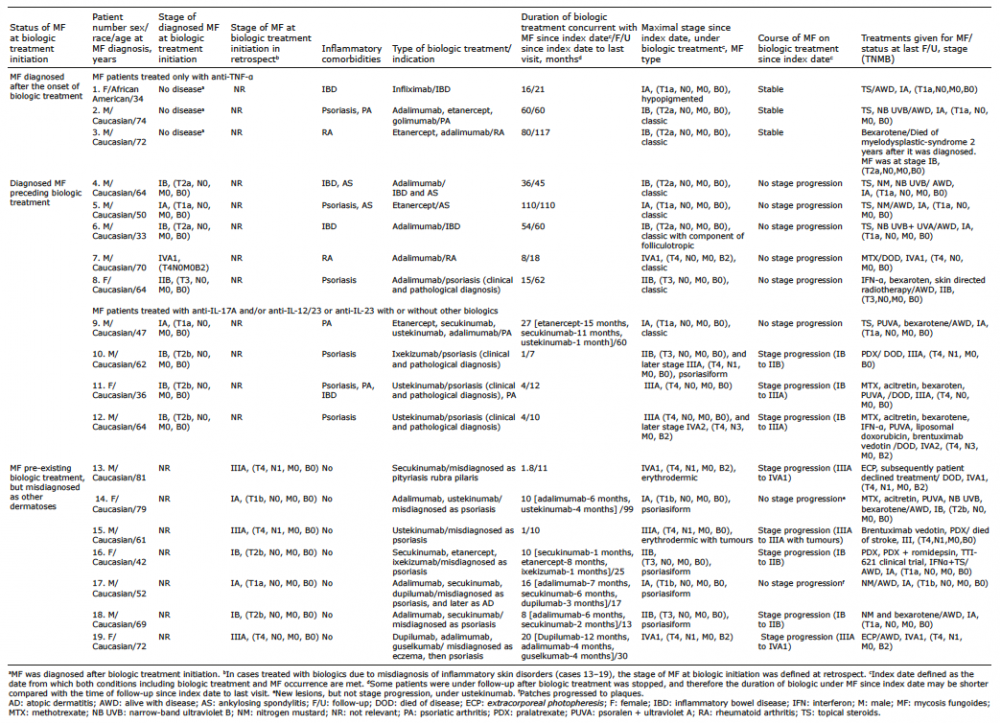
Table I. Demographic and clinical data for patients with mycosis fungoides (MF), treated with biologics
1Division of Dermatology and 10Institute of Pathology, Rabin Medical Center – Beilinson Hospital, Petach Tikva, 2Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel, 3Department of Dermatology, University Hospital Zürich & Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland, 4Department of Dermatology, 12 de Octubre Hospital, CIBERONC, Institute i+12, Medical School, University Complutense, Madrid, Spain, 5Department of Dermatology, Johns Hopkins Medicine, Baltimore, MD, USA, 6Department of Dermatology, Columbia University, New York, NY, USA, 7Department of Dermatology, Andreas Sygros Hospital, 8Department of Dermatology, Attikon General Hospital, University of Athens Medical School, Athens, Greece, 9Department of Dermatology, Sheba Medical Center, Ramat-Gan, Israel, and 11Cutaneous Lymphoma Program, Department of Dermatology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
#These authors contributed equally to this work.
Literature regarding the effect of biologics on the course of mycosis fungoides (MF) is scarce. This multi-centre study analysed retrospective data on 19 patients with MF, who were treated with biologics; 12 for inflammatory conditions coexisting with MF, and 7 for MF misdiagnosed as an inflammatory skin disease. Eight patients were treated with anti-tumour necrosis factor-α-monotherapy; 6 had early-stage MF, in 3 patients MF preceded and in 3 MF was diagnosed after initiation of biologics, with no stage-progression or with stable disease, respectively (median treatment time concurrent with MF 57 months). Two patients had advanced stage MF: IIB, treated for 15 months with no stage-progression, and IVA1, treated for 8 months, died of disease 10 months later. The other 11/19 patients received anti-interleukin-17A and/or anti-interleukin-12/23 or anti-interleukin-23 (with/without anti-tumour necrosis factor-α/anti-interleukin-4/13), with stage-progression in 8 patients after a median of 8 months’ treatment. Although, in general, biologics should be avoided in patients with MF, these results indicate that anti-tumour necrosis factor-α-monotherapy might not aggravate the disease course in early-stage patients. Interleukin-17A, interleukin-12/23 and interleukin-23 pathway-blockers may prompt progression of MF.
Key words: cutaneous T cell lymphoma; mycosis fungoides; biologic treatment; anti-TNFα; anti-IL-12/23; anti-IL23; anti-IL17A.
Accepted Sep 18, 2020; Epub ahead of print Sep 23, 2020
Acta Derm Venereol 2020; 100: adv00277.
doi: 10.2340/00015555-3642
Corr: Iris Amitay-Laish, Division of Dermatology, Rabin Medical Center – Beilinson Hospital, Petach Tikva 4941492, Israel. E mail: amitaylaishiris@gmail.com
Presented at the European Organisation for Research and Treatment of Cancer (EORTC), Cutaneous Lymphoma Task Force Meeting, Athens, in September 2019.
Since, almost as a rule, the treatment of patients with inflammatory conditions with biologics is terminated on diagnosis of mycosis fungoides, information on the course of mycosis fungoides under biologic treatment is scarce. Analysis of real-life data for 19 patients with mycosis fungoides being treated with biologics, revealed that anti-tumour necrosis factor-α-monotherapy may not always adversely affect early-stage disease. In contrast, in the vast majority of patients with mycosis fungoides, continuation of treatment with interleukin-17A, -12/23, and -23 pathway-blockers led to prompt progression of the disease. These observations may guide clinicians in considering the advantages and disadvantages in continuation of tumour necrosis factor-α-blockers in the rare co-occurrence of early-stage mycosis fungoides and inflammatory conditions.
The introduction of anti-tumour necrosis factor-α (TNF-α), as well as newer biologics, including anti-interleukin (IL)-17, anti-IL-12/23 and anti-IL-23, has revolutionized the management of autoimmune and inflammatory diseases (1–3). Patients with psoriasis, one of the most common inflammatory dermatoses, affecting 2–4% of the population in Western countries, now receive biologic treatment sooner in the course of their disease, following treatment with fewer conventional agents (3).
The risk of lymphoma under treatment with biologics, mainly anti-TNF-α, has been investigated and debated extensively (4–15). According to the US Food and Drug Administration (FDA) Adverse Event Reporting System, there was an increase in the risk of T-cell non-Hodgkin lymphomas, mainly mycosis fungoides (MF)/Sézary syndrome (SS), with anti-TNF-α combined with thiopurines or thiopurines alone, but not with anti-TNF-α alone (4).
Specific reviews of the literature yielded approximately 70 cases of MF and SS, in addition to 20 cases of other less common cutaneous T-cell lymphomas (CTCLs), under treatment with biologic agents, mostly anti-TNF-α and a few cases under treatment with anti-IL-12/23 or anti-IL-17 (11–25). The median interval from initiation of biologic treatment to diagnosis of CTCL was 5.5–32 months (11–13). Some patients, when information was provided, were also treated with thiopurines or cyclosporine (12, 16, 22, 23), which may also aggravate the course of lymphoma (4, 26).
Overall, there are 2 settings in which MF/SS has been reported under biologic treatment, with approximately equal prevalence. In the first setting, patients were treat-ed with biologics for a chronic inflammatory condition, such as inflammatory bowel disease (IBD) or rheumatoid arthritis (RA), and during the course of treatment, were diagnosed with MF/SS (12, 13, 20–22, 24, 25).
In the second setting, patients were treated with biologics for misdiagnosed psoriasis, atopic dermatitis, eczema, or idiopathic erythroderma, and were subsequently correctly diagnosed with MF while under treatment with biologics (11–16, 23, 24).
In almost all cases in both settings, biologic treatment was terminated on diagnosis of MF/SS. In a few patients, all with an inflammatory comorbidity, the treatment was continued despite the diagnosis of MF, after considering its advantages and disadvantages (11–25).
Therefore, direct information is still lacking on the course of MF under biologic treatment.
The aim of the present study was to evaluate the effect of biologics on the course of MF.
Setting and patients
Data were retrospectively collected on all patients fulfilling the following inclusion criteria: patients who had MF while under treatment with any type of anti-TNF-α, and/or anti-IL-17, and/or anti-IL-12/23, and/or anti-IL-23 agent/s, for any time frame, and were managed at the following institutes: Cutaneous Lymphoma Clinics, Rabin Medical Center, Israel (from January 2009), University of Pittsburgh, USA (from January 2013), University Hospital Zürich, Switzerland (from November 2011), Hospital Universitario 12 de Octubre, Spain (from June 2003), Johns Hopkins Medicine, USA (from January 2016), and Andreas Sygros or Attikon General Hospital, Greece (from January 2011). The study endpoint, for all sites, was June 2019.
This cohort included 3 groups of patients. The first group comprised patients who, during treatment with systemic biologic/s for an inflammatory disease, including: IBD, RA, psoriasis, or ankylosing spondylitis (AS), developed MF. This group included patients only if biologic treatment was continued for any time-period. The second group comprised patients with MF diagnosed before biologic treatment, which was given for inflammatory comorbidities; (IBD, arthritis, etc.). The third group comprised patients with MF presenting prior to the biologic treatment, but misdiagnosed as other dermatoses, for which the biologic treatment was given.
Of note, in the first 2 groups, treatment with biologics was continued due to significant inflammatory comorbidity, after careful consideration of the pros and the cons, while in the third group once misdiagnosis was recognized, biologic treatment was discontinued.
Patients also treated with other immunosuppressive medication/s (azathioprine or cyclosporine) were excluded.
MF was defined according to the World Health Organization – European Organization for Research and Treatment of Cancer (WHO–EORTC) classification of cutaneous lymphomas (27). Staging was determined using the tumour node metastasis (TNM)system (28), and stage progression was defined according to the International Society for Cutaneous Lymphomas (ISCL), the United States Cutaneous Lymphoma Consortium (USCLC), and the EORTC (29).
Demographic, background, and disease-related parameters were recorded, as follows: sex, race, age at diagnosis of MF, inflammatory comorbidities, the indication for biologic treatment, type of biologic agent/s prescribed, duration of MF under biologic treatment since the index date (i.e. the date from which both conditions, including biologic treatment and MF occurrence, met); type of MF, TNMB staging at initiation of biologic treatment, most advanced stage and stage at last follow-up, treatment received for MF, and outcome. Follow-up data were assessed from the index date to the last follow-up. As some of the patients continued follow-up after cessation of the biologic treatment, the duration of biologic treatment concurrent with MF from the index date may have been shorter than the duration of follow-up from the index date to the last visit.
The study was approved by the local Institutional Review Committee of Rabin Medical Center.
Table I summarizes the clinical characteristics of the patients, types of biologics given, the course of MF on biologics, and duration of follow-up. Fig. 1 shows the status/stage of MF at initiation and during the course of biologic treatment, the type of biologic agent given, and the patients showing stage progression.

Table I. Demographic and clinical data for patients with mycosis fungoides (MF), treated with biologics
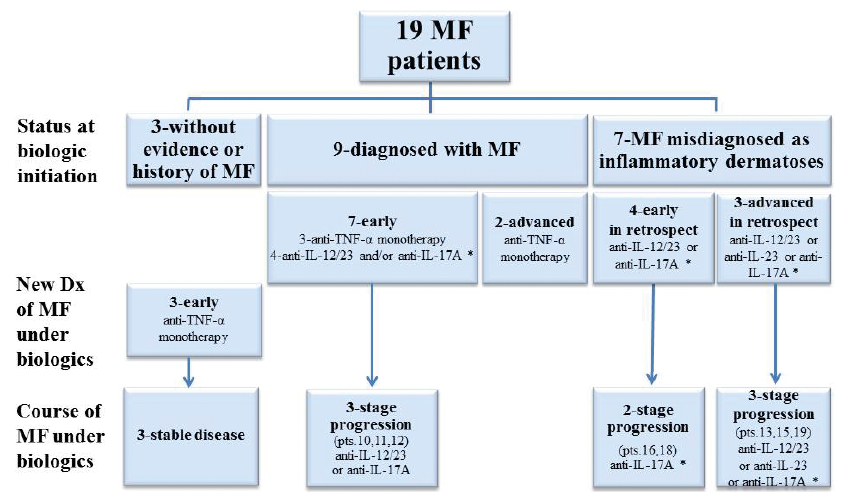
Fig. 1. Status and stage of mycosis fungoides (MF) at initiation and during the course of biologic therapy. *Some were also treated with other biologics. anti-TNF-α: anti-tumour necrosis factor-α; IL: interleukin; pts: patients; Dx: diagnosis.
The cohort included 19 patients (13 males, 6 females; 18 Caucasians and 1 African-American). Median age at diagnosis of MF was 64 years (range 33–81 years). In 12 patients (numbers 1–12), biologics were given for a true inflammatory comorbidity, including: biopsy proven psoriasis in 4 patients (also in 1 with psoriatic arthritis), and psoriatic arthritis, RA, IBD, and AS in 8 patients, as shown in Table I. In the other 7 patients (numbers 13–19), biologics were given for an initial misdiagnosis of another dermatosis.
Sixteen patients (numbers 4–19) had MF at initiation of biologic treatment, diagnosed either before treatment onset or in retrospect: 11 early-stage disease (IA/IB) and 5 advanced-stage disease (1-IIB, 3-IIIA, 1-IVA1). They were followed for a median of 10 months (range 1–110 months) from the index date, while being treated with biologic therapy. Eight patients (50%) had stage progression during follow-up (numbers 10–13, 15, 16, 18, 19). By the last follow-up (median 22 months, range 7–110), 5 had died of disease: 4 (numbers 10–13) after progression of MF under biologics, and 1 with stage IVA1 MF (number 7), diagnosed at this stage before biologic treatment was initiated.
In the other 3/19 patients (numbers 1–3), MF was diagnosed after the onset of biologic treatment. Administration of biologic agents with concurrent MF was continued for 16, 60 and 80 months. All 3 had early-stage MF and a stable disease course. By the last follow-up, one patient had died of myelodysplastic syndrome (number 3).
Subanalysis of the course of mycosis fungoides by type of biologic treatment
Eight patients were treated only with anti-TNF-α, (numbers 1–8). Of these, 6 had early-stage MF: 3 before biologic treatment initiation and 3 were diagnosed as early-stage MF in the course of biologic treatment. All 6 patients received mostly skin-targeted therapies for the underlying disease (Table I) and responded well, as expected. The 3 patients with MF pre-existing biologic treatment (1-IA, 2-IB) did not progress to higher stages during continuation of anti-TNF-α, and all 3 patients with MF newly diagnosed after initiating anti-TNF-α (1-IA, 2-IB), had stable early-stage disease while continuing biologic treatment. The median duration of treatment of anti-TNF-α from the index date was 57 months (mean 59 months, range 16–110). At the last follow-up (median 60 months, range 21–117), 5 patients were alive with early-stage MF and 1 had died of myelodysplastic syndrome. The remaining 2/8 patients who received anti-TNF-α-monotherapy (numbers 7, 8) had advanced MF at treatment initiation. They were treated for 8 and 15 months, respectively. Patient 7, with stage IVA1 MF, died of disease 10 months after stopping treatment with anti-TNF-α and patient 8, with stage IIB, did not progress.
Eleven patients received anti-IL-17 and/or anti-IL-12/23, or anti-IL-23 agent/s, with/without anti-TNF-α and/or anti-IL4/13, for a median of 8 months (range 1–27) (numbers 9–19). Eight had early-stage MF at initiation of biologic therapy and 3 had advanced-stage MF. The indication for biologics was histologically confirmed psoriasis in 3 patients (numbers 10–12), in one also with psoriatic arthritis, and psoriatic arthritis in another (number 9). In the other 7 patients, biologic treatment was given for a presumed diagnosis of psoriasis, eczema, atopic dermatitis, or pityriasis rubra pilaris, and it was stopped when the diagnosis of MF was established. Stage progression was noted in 8/11 patients (Fig. 1, Table I): 3 patients with early-stage disease IB exhibited newly developed tumours (numbers 10, 16, 18; Figs 2 and 3), 2 patients with early-stage IB disease developed erythro-derma (numbers 11, 12), and 3 with advanced-stage disease exhibited either blood involvement (stage B2; numbers 13, 19) or tumours in the context of erythroderma (number 15). Of note, in all 3 patients who were at stage IA MF at initiation of biologic treatments, stage progression according to the formal definition (29) was not recorded. However, in one patient (number 14) more lesions were recorded, and in another (number 17), some of the patches developed into plaques (Table I).
The median follow-up time for these patients was 13 months (range 7–99). At the last follow-up, 6/11 patients were alive with disease: 2 were down-staged to sustained stage IA, 2 remained at stage IA, one progressed from stage IA to IB, and one was at stage IVA1. The other 5 patients died, 4 of MF and one of stroke.
Of note, 2 patients at some point during their manage-ment were treated with anti-IL4/13- dupilumab for a presumed diagnosis of atopic dermatitis (numbers 17, 19), but this proved ineffective and was discontinued after 3 and 12 months, respectively.
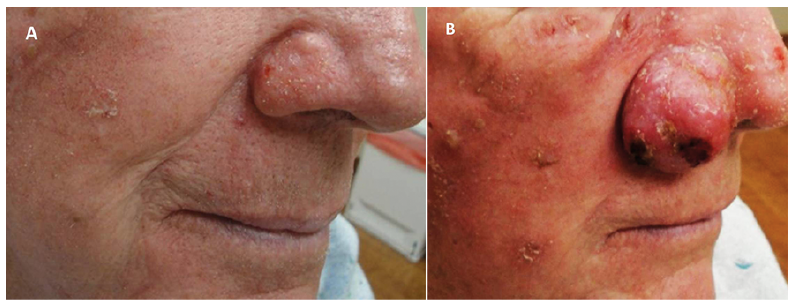
Fig. 2. Patient 10. Findings before and after treatment with ixekizumab. (A) Ill-defined erythemas on the cheek and nose before biologic treatment. (B) Appearance of a tumour on the ala nasi after biologic treatment.
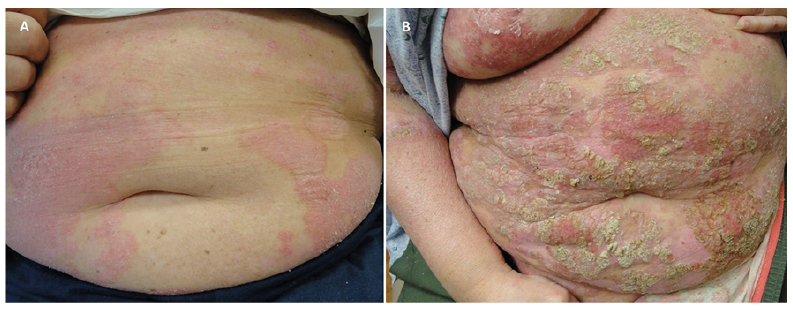
Fig. 3. Patient 16. Findings before and after treatment with secukinumab, etanercept, and ixekizumab. (A) Well-defined erythematous scaly plaques on the trunk, initially diagnosed as psoriasis, before biologic treatment. (B) Widespread plaques, some infiltrated, covered by yellowish thick scale-crusts on the trunk, after biologic treatment.
This multicentre study adds considerably to the growing body of literature on the course of MF under biologics. Our experience suggests that the course of MF under treatment with biologic agents is not uniform. On the one hand, anti-TNF-α-monotherapy did not adversely affect the course of early-stage MF. On the other hand, rapid stage progression was noted in most of the patients treated with anti-IL-17 and/or anti-IL-12/23 agents.
Among the 6 patients with early-stage MF treated with anti-TNF-α for an inflammatory comorbidity, 3 had pre-existing MF and showed no stage progression during treatment. In the other 3 patients, early-stage MF was diagnosed after anti-TNF-α was started. Although we cannot rule out the possibility that, in these cases, MF was caused by the biologic treatment, continued treatment with anti-TNF-α did not result in further stage progression. Most of these patients received conventional skin-targeted agents for the early-stage MF and responded as expected (Table I).
In keeping with the findings of the current study, an earlier report, based on 2 French national registries, described 3 patients who had what was defined as limited MF while under biologic treatment, (infliximab; personal communication), for IBD or RA. All 3 achieved complete or partial remission after local treatment while continuing the biologics (13). Likewise, others reported that most patients diagnosed with MF while being treated with anti-TNF-α for an inflammatory systemic comorbidity were diagnosed at an early stage and had an indolent outcome (11–25). Tsimberidou et al. (30) described 2 patients with stage IB MF in whom partial remission or minor improvement were noted under treatment with etanercept.
The present study does not provide sufficient information on the course of advanced-stage MF under anti-TNF-α. Only 2 of our patients had advanced-stage MF, 1 of whom died of the disease. Interestingly, of the ~60 patients previously reported in the context of MF/SS and anti-TNF-α, ~35% were diagnosed at an advanced stage (11–25). Based on these patients, some of the authors concluded that the anti-TNF-α treatment may have aggravated the course of MF. However, most of these patients were given anti-TNF-α to treat inflammatory skin disorders, such that the possibility of an underdiagnosis of pre-existing advanced MF in at least some of them cannot be excluded.
Most of the literature on MF and biologics focuses on anti-TNF-α, and data on the effect of anti-IL-17 and/or anti-IL-12/23 on MF are very limited (11–14). In the present cohort, of the 11 patients treated with anti-IL17 and/or anti-IL-12/23, or anti-IL-23, 8 patients, all with at least stage IB, showed stage progression under treatment. They included 5 patients in whom MF was initially misdiagnosed as another dermatosis (Table I). Likewise, in all 6 previously reported cases of MF under treatment with anti-IL-17 and/or anti-IL-12/23, the biologic/s were given for a cutaneous indication, and most of the patients were subsequently diagnosed with advanced-stage MF (11, 12, 14). Yoo et al. (11) described 2 patients with a long history of diagnosed psoriasis who were subsequently diagnosed with stage IB and stage IIIA MF after 12 and 8 weeks’ treatment, respectively, with secukinumab (11). Others described patients with psoriasis/presumed psoriasis who were ultimately diagnosed with advanced-stage MF under treatment with etanercept and ixekizumab (n = 1) (14) or with stage IIB MF, SS, or primary cutaneous epidermotropic CTCL (n = 1 each) under treatment with ustekinumab plus anti-TNF-α (12).
These cases, together with ours, suggest that anti-IL-17 and anti-IL-12/23 agents may aggravate MF. However, as most patients were initially misdiagnosed with other dermatoses, the stage of MF at initiation of biologic treatment is unclear.
The previously reported cases (11–25), together with our series, highlight the extreme caution that should be taken before initiating biologics for a presumed dermatosis, as MF is notorious for being a great imitator of cutaneous benign inflammatory diseases (31). Confirmatory skin biopsy prior to initiation of biologics is not a standard of care and is not performed routinely. The issue is particularly complicated in cases of psoriasis, as MF may mimic psoriasis both clinically and pathologically (12, 26, 31). The additional presence of alopecia, induration, erosions/ulcerative lesions should raise the suspicion of MF (31). Furthermore, some patients with MF may have concomitant psoriasis, as occurred in 6 patients in the present cohort. Longitudinal follow-up, repeated biopsies, and clone comparison may help to arrive at the correct diagnosis.
The pathomechanism underlying the seemingly differential effect of the various classes of biologics on the course of MF is unclear. The few studies of the TNF-α pathway in this context suggest that it plays a complex role in the pathophysiology of MF (30, 32–34). MF
tumourigenesis was found to be associated with changes in the regulation of a combination of anti-apoptotic signalling through different TNF receptors (32). TNF has also been implicated in the development of CTCL by virtue of its ability to promote epidermotropism via induction of interferon-inducible protein. In addition, anti-TNF antibodies down-regulated NF-kB and inhibited CTCL growth in cell culture (33, 34). These findings may provide a theoretical explanation for the “non-harmful” effect of anti-TNF on the course of 6 patients with early-stage MF in the present cohort.
Studies of the role of IL-17 in MF have yielded contradictory results (11, 35–38). Some have shown that IL-17 is highly expressed in a subset of patients with MF and is associated with progressive disease (35, 36), whereas others have reported low IL-17 levels in patients with MF (37, 38). Regarding the tumour-related immune response in general, there are several lines of evidence based on human and mouse tumours suggesting that TH-17 cells can promote protective anti-tumour responses. TH-17 cells were found to be negatively correlated with the presence of TReg cells and positively correlated with effector immune cells, including IFNγ+ effector and cytotoxic CD8+ T cells, and natural killer cells, in the same tumour microenvironment (39).
Regarding the IL-12 pathway, which was blocked in 5 of the patients in the current stduy treated with ustekinumab, IL-12 is a powerful inducer of IFN-γ production and was shown to augment natural killer cell cytotoxicity and cytotoxic T-cell proliferation and function, all of which may mitigate the Th2 skewing in advanced CTCL (40).
Together, these data suggest that TH-17 or IL-12 blockade may lead to disease progression.
Study limitations
The limitations of this study include the retrospective design, small cohort, and relatively limited long-term follow-up. In addition, further stage progression under biologics in patients initially misdiagnosed with advanced-stage MF, might be, to some extent, due to the fact that these advanced patients were untreated for MF, and not merely due to the biologic treatment.
Conclusion
Several conclusions may be drawn from this study. First, before considering biologics for benign cutaneous inflammatory disorders, clinicians should re-think the indication, take a second look for clinical clues of MF, revise the histology or take another biopsy, and consider blood assessment, including flow cytometry. Secondly, although similar to other immunosuppressant treatments, biologics in general should be avoided in cases of MF. Nevertheless, based on our experience, together with the few cases reported in the literature, it seems that anti-TNF-α may not always adversely affect the course of unequivocal early-stage MF, and the pros and cons of anti-TNF-α-therapy should be considered on a case-by-case basis. Further studies are needed to search for a biomarker to assist in predicting the risk of MF aggravation under anti-TNF-α or other biologics. Thirdly, we found that anti-IL-17 and/or anti-IL-12/23 or anti-IL-23treatment/s were associated with rapid aggravation of diagnosed and undiagnosed MF in several patients, all with at least stage IB MF. Whether the course of unequivocal classic very early-stage MF-IA might also progress under these treatments is not known and requires further study. A large international observational study is needed to fully clarify the complex relationship between MF and biologic agents and to guide clinical decisions.
The authors have no conflicts of interest to declare.