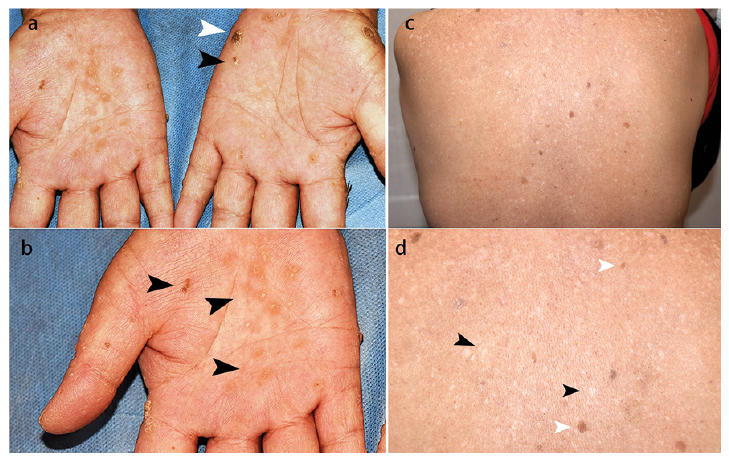
A 56-year-old woman presented with multiple indurated papules on both hands and feet for 15 years. The lesions had started as yellowish dotted papules on her palms and soles and had progressively enlarged and increased, with itching. No pain or ulceration was noted. She also noticed skin discolouration during the past 10 years, especially on the trunk, without any symptoms. The patient had a history of asthma for over 40 years and had taken traditional Chinese medicine containing realgar for years. She had stopped these remedies many years before she started to notice the skin lesions. Clinical examination revealed multiple keratotic plaques and verrucous papules with
yellowish crusting on her palms and soles (Fig. 1a, b). Guttate hypopigmented macules and hyperpigmented patches were noted on her trunk (Fig. 1c, d). A systemic review of the cardiovascular system, gastrointestinal system, neuro-logical system, respiratory system, genitourinary system, and the haematological system was unremarkable. A
cutaneous biopsy was taken of a keratotic plaque on the left palm (Fig 1a, white arrowhead).
What is your diagnosis? See next page for answer.
Acta Derm Venereol 2020; 100: adv00279.
Diagnosis: Squamous cell carcinoma secondary to arsenic keratosis
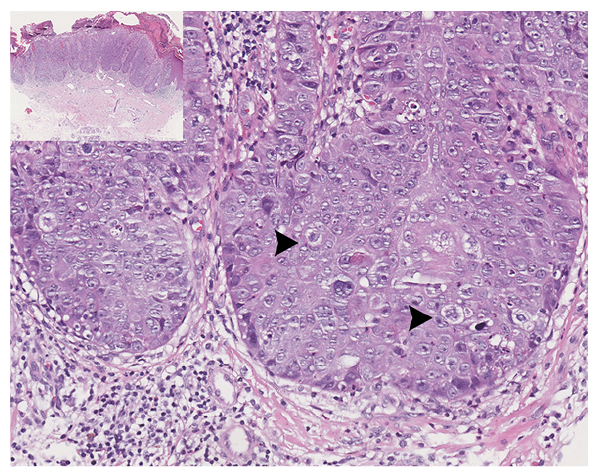
The skin biopsy revealed epidermal hyperplasia with atyp-ical keratinocytes and remarkable mitoses involving the full-thickness of the epidermis, consistent with squamous cell carcinoma (SCC) in situ (Fig. 2). Given her long history of taking herbal medicine containing realgar, which is a
monoclinic arsenic sulphide mineral, combing with the typical guttate hypopigmentation and palmoplantar hyperkeratotic lesions, a diagnosis of SCC and arsenic keratosis secondary to chronic arsenic poisoning was made. The lesion of SCC on the palm was treated successfully with topical imiquimod within 12 weeks. Oral acitretin was initiated to control arsenic keratosis.
Arsenic has been classified as a class I human car-cinogen, which exists mostly as an inorganic form or less toxic organic form in water, food, and occupation source (1). Arsenic sulphide, or realgar, has a long history of use in traditional Chinese herbal formulae to treat psoriasis, syphi-lis, asthma, joint pain, epilepsy and cancer decades ago (2–4). Contaminated drinking water is another frequently reported source of arsenic exposure (5). Prolonged use of mineral arsenicals resulted in chronic toxicity involving multi-systems. Dermatological changes are the common feature and often serve as the initial sign of chronic arsenic poisoning. The diagnosis is often based on the corn-like or verrucous keratinized papules on the palmoplantar region, and diffuse pigmentation or spotted hypopigmentation on the trunk and limbs, with a characteristic “raindrop” appearance. Skin cancer may arise in pre-existing arsenical keratoses and may be aggressive. Chronic exposure to ar-senic poses an increased risk for developing various types of skin cancers, such as SCC, basal cell carcinoma, and other non-melanoma skin tumours with long latencies of up to 50 years (6). Arsenic levels in blood, urine, hair, and nails have been used to assess chronic exposure to arsenic (7). Arsenic poisoning involves not only the skin, but almost all the other organ systems. Peripheral neuropathy with a glove and stocking anaesthesia, cardiac arrhythmias and cardiomyopathy, chronic diarrhoea and vomiting, as well as neutropaenia are the most frequent systemic symptoms. The most serious consequence is the malignant change in almost all organs of the body. Therefore, systemic reviews should be performed on patients with skin lesions indicating chronic arsenic exposure.
Arsenic is deposited in the keratin-rich tissues: nails, hair, and skin. Arsenic accumulates in the skin through binding epidermal keratin that is enriched with thiols. Under the influence of oxidative stress, chromosomal abnormality, oncogene activation, p53 mutation, altered transcription factor and growth factors levels, impaired DNA repair, G2/M cell cycle arrest, as well as immune dysfunction, arsenic keratosis may occur in carcinogenesis (1, 8).
Effective treatment of chronic arsenic toxicity is not yet established. Therefore, the first step in treating patients is to prevent further exposure to arsenic. Retinoids repress abnormal differentiation and proliferation of keratinocytes. Vitamins C and E antagonize the oxidative damage caused by arsenic poisoning, and selenium enhances the excretion of arsenic (9). For multiple severe keratotic papules or nodules that cannot be excised, imiquimod ointment is a safe and effective therapy. Lesions with a sudden increase in size, cracks, and bleeding, and those of Bowen’s disease, or SCC, must be surgically excised at the earliest opportunity.
This case underscores the need for close follow-up of patients with a history of medicinal arsenic exposure and highlights the importance of the recognition of the charact-eristic skin lesions for arsenic poisoning, which may lead to timely treatment of the associated aggressive malignancies.