REVIEW ARTICLE
Metformin: A Potential Treatment for Acne, Hidradenitis Suppurativa and Rosacea
Minah CHO, Yu Ri WOO, Sang Hyun CHO, Jeong Deuk LEE and Hei Sung KIM
Department of Dermatology, Incheon St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
Metformin is a widely used drug for treatment of diabetes mellitus, due to its safety and efficacy. In addition to its role as an antidiabetic drug, numerous beneficial effects of metformin have enabled its use in various diseases. Considering the anti-androgenic, anti-angiogenic, anti-fibrotic and antioxidant properties of metformin, it may have the potential to improve chronic inflammatory skin diseases. However, further evidence is needed to confirm the efficacy of metformin in dermatological conditions, This review focuses on exploring the therapeutic targets of metformin in acne vulgaris, hidradenitis suppurativa and rosacea, by studying their pathogeneses.
Key words: acne vulgaris; hidradenitis suppurativa; metformin; rosacea.
SIGNIFICANCE
Metformin is a well-known antihyperglycaemic medication, but recent studies indicate that its benefits may extend beyond treatment of diabetes mellitus. Metformin is also used in the treatment of a variety of conditions, including obesity and polycystic ovary syndrome (PCOS), and in cancer prevention. Regarding the skin, metformin is a promising therapeutic option for acne, hidradenitis suppurativa and rosacea. The action of metformin is thought to be due to the drug improving insulin sensitivity, modulating androgen output, lowering oxidative stress, inflammation, and scarring, and improving blood vessel health. This review discusses these effects.
Citation: Acta Derm Venereol 2023; 103: adv18392. DOI https://doi.org/10.2340/actadv.v103.18392.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Accepted: Nov 8, 2023; Published: Dec 11, 2023
Corr: Hei Sung Kim, Department of Dermatology, Incheon St Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. E-mail: hazelkimhoho@gmail.com
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Metformin, a biguanide derivative, is a drug primarily prescribed for diabetes mellitus (DM). Due to its safety and efficacy, metformin has been used in type 2 DM since the 1950s (1). Recent studies report that metformin can be used for diseases other than DM, such as cancer, obesity, liver diseases, and cardiovascular diseases (2). In dermatology, the use of metformin in hirsutism, acne, hidradenitis suppurativa (HS), acanthosis nigricans, psoriasis, and skin cancer is showing promising results (3, 4). However, the underlying mechanism is not fully elucidated. Anti-hyperglycaemic effects, anti-androgenic effects, antioxidant effects, anti-fibrotic effects, anti-proliferative effects, anti-angiogenic effects, and pro-autophagic effects have been suggested as possible mechanisms by which metformin acts in these skin disorders (2, 3, 5). This review focuses on the dermatological potential of metformin in acne vulgaris, HS and rosacea, based on its mechanism of action.
ANTI-ANDROGENIC EFFECTS OF METFORMIN
Hyperandrogenism in skin diseases
Androgens are well-known hormones that affect many organs, including the skin. Testosterone and its more potent reduced form, 5α-dihydrotestosterone (DHT), can bind to androgen receptors found in sebocytes, dermal papilla cells, the outer root sheath of hair follicles, sweat glands, endothelial cells and keratinocytes (6). Weak prohormones, such as dehydroepiandrosterone (DHEA) and androstenedione, can also be converted to testosterone and DHT in sebocytes, sweat glands and dermal papilla cells (7). Androgen and androgen receptors are thought to be involved in the pathogenesis of a number of skin diseases, including androgenetic alopecia, acne vulgaris, HS, hirsutism and acanthosis nigricans, by influencing hair growth, sebaceous gland proliferation, sebum production, and epithelialization (7, 8).
Acne vulgaris is a disorder of the pilosebaceous unit common in adolescents and young adults. Its major pathogenic factors include follicular hyperproliferation, sebum production, Cutibacterium acnes (C. acnes) and local inflammation. Hormonal dysregulation also plays a role, especially androgens, which increase sebum production. Androgens regulate the function of sebaceous glands by binding to androgen receptors on sebocytes (9). Insulin-like growth factor-1 (IGF-1) stimulates androgen receptor signalling by reducing nuclear forkhead box-O1 (FoxO1) transcriptional activity via activating the phosphoinositol-3-kinase (PI3K)-Akt pathway (10). Eventually, the mammalian target of rapamycin complex 1 (mTORC1) signalling pathway is activated to increase lipogenesis by upregulating sterol regulatory element-binding proteins (SREBPs), which results in hyperseborrhoea in patients with acne (11). C. acnes, which favours sebum-rich condition is also involved in acne pathogenesis (12). IGF-1 triggers inflammatory cytokine responses in acne by upregulating Toll-like receptor (TLR) 2/4 expression in sebocytes (13). In this regard, acne is exacerbated under conditions in which IGF-1 is elevated, such as a high glycaemic load diet, milk consumption and polycystic ovarian syndrome (PCOS) (14).
HS is a chronic inflammatory disorder of hair follicles and is characterized by deep-seated nodules, abscesses, fistulae, sinus tracts, and hypertrophic scars in intertriginous and anogenital regions of the body (15). Its comorbidities and risk factors include smoking, obesity, metabolic syndrome, cardiovascular disease, diabetes and inflammatory bowel disease. Although the pathogenesis of HS is yet to be fully elucidated, follicular hyperkeratosis, ruptured hair follicles, and subsequent inflammation of the pilosebaceous-apocrine unit play a key role. Similar to other inflammatory skin diseases, immune dysregulation plays an important role in the process. Androgens have been implicated in HS, since premenstrual exacerbation is often seen in female patients (16). Some patients with HS carry high concentrations of total testosterone and free androgen index scores (17), but the majority of patients have normal serum androgen levels (16, 18). Thus, it is speculated that the peripheral conversion of androgens is increased in patients with HS. However, a study that investigated androgen metabolism in apocrine glands found that the activity of the 5α-reductase enzyme catalysing DHT synthesis from testosterone was similar in patients with HS and healthy controls (19). In spite of normal androgen profiles in patients with HS, improvement in HS has been reported following antiandrogen treatments, including spironolactone, finasteride, and cyproterone acetate (20–24). As in acne vulgaris, mTORC1 was increased in both the lesional and non-lesional skin of patients compared with healthy controls (25). Considering that patients with HS have a high prevalence of obesity and metabolic diseases which are characterized by hyperandrogenism, insulin resistance, and mTORC1 upregulation, androgen may play a role in the pathogenesis of HS. Further studies are needed to investigate the detailed underlying mechanisms.
Potential anti-androgenic targets of metformin
Metformin activates adenosine monophosphate-activated protein kinase (AMPK) to increase the expression of glucose transporter 4 (GLUT4) and the translocation of GLUT4 to the plasma membrane (26). This pathway facilitates peripheral glucose utilization, enhances insulin sensitivity, and reduces IGF-1 levels (4, 26). Decreased IGF-1 levels increase FoxO1 availability and, eventually, suppress androgen receptor signalling. Metformin downregulates mTORC1, which is activated by IGF-1 in patients with acne vulgaris and HS, in an AMPK-independent manner (27). In particular, the anti-androgenic effects of metformin have been reported in patients with PCOS. Metformin decreases free-bioactive IGF-1 by increasing insulin-like growth factor-binding protein (IGFBP)-1 in patients with PCOS (28). Metformin also suppresses the TLR4-associated nuclear factor kappa B (NF-κB) signalling pathway, which is positively regulated by hyperandrogenism (29). It also represses testosterone-induced androgen receptor expression in cells (30). The anti-androgenic effects of metformin suggest its effectiveness in diseases associated with hyper-androgenism and insulin resistance, including PCOS, HS, acne vulgaris, hirsutism and obesity.
ANTI-ANGIOGENIC EFFECTS OF METFORMIN
Angiogenesis in skin diseases
Angiogenesis is the formation of new blood vessels from pre-existing blood vessels. It is essential for physiological repair, including wound healing, by increasing perfusion, nutrient supply, and the elimination of waste products (31, 32). However, excessive angiogenesis may play a role in various cutaneous diseases, including psoriasis, atopic dermatitis, rosacea, haemangiomas, melanoma, and non-melanoma skin cancer (32–34). Angiogenesis is triggered by the release of angiogenic factors, such as vascular endothelial growth factors (VEGFs), fibroblast growth factors (FGFs), angiopoietins (Ang1 and Ang2), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF-β), by hypoxic tissue (31–36). Furthermore, the process is facilitated by the degradation of the basement membrane and extracellular matrix (ECM) by matrix metalloproteinases (MMPs). MMPs, especially MMP-9, promote angiogenesis by activating VEGF (35, 37, 38). Since immune cells are a major source of these angiogenic factors, angiogenesis may accompany chronic inflammatory skin diseases. Specifically, neutrophils produce VEGF-A and MMPs. Mast cells and macrophages release VEGF-A and VEGF-B and T-helper type 17 (TH17) cells produce interleukin (IL) 17, which enhances angiogenesis by increasing VEGF-A (39).
Rosacea is a chronic inflammatory disease that predominantly affects the central face. Although its aetiology remains elusive, the inflammatory pathway includes the dysregulation of innate and adaptive immune systems and neurovascular dysregulation. Triggering factors, such as heat, ultraviolet (UV) radiation, emotional stress, microorganisms, alcohol and other irritants, may initiate the inflammatory pathway via a number of mediators. TLR2 recognizes several trigger factors and is upregulated in patients with rosacea. TLR2 induces the activation of cathelicidin LL-37 via kallikrein 5 (KLK5), leading to erythema, telangiectasia and angiogenesis by releasing inflammatory cytokines, chemokines, proteases and angiogenic factors (40, 41). TLR2 also activates the NLR family pyrin domain-containing 3 (NLRP3) inflammasome, which induces IL-1β and tumour necrosis factor (TNF), mediating inflammation and prostaglandin E2 synthesis (42). Protease-activated receptor 2 (PAR2), expressed by keratinocytes, endothelial cells and innate and adaptive immune cells, acts as a key mediator of inflammation in rosacea. PAR2 also interacts with TLR2, and KLK5 further activates PAR2 signalling pathways (43, 44). Following the activation of TLR2, PAR2 and the NLRP3 inflammasome, T-helper type 1 (TH1) cells, TH17 cells, macrophages and mast cells release angiogenic factors, such as VEGF, TGF-β, IL-17 and MMPs, in patients with rosacea (34, 44, 45).
The key proinflammatory cytokines of HS include TNF-α, IL-1β, and IL-17 (15, 46). TNF-α released by keratinocytes and activated dendritic cells induces defective insulin signalling by activating adipocytes and muscle cells (47, 48). It also increases TH17 cells, resulting in elevations of IL-17 levels. IL-1β combined with TNF-α activates MMP2 and MMP9 (46). IL-17 induces the expression of the NLRP3 inflammasome in neutrophils and macrophages, further enhancing inflammatory responses by increasing TNF-α, IL-1β, caspases and MMPs (15, 46). The role of angiogenesis in HS has yet to be elucidated, but activation of the NLRP3 inflammasome, MMP 9 overexpression, and elevated levels of TNF-α, IL-1β and IL-17 are known to act as pro-angiogenetic factors in other inflammatory skin diseases, such as rosacea. IL-1α, one of the keratinocyte-derived proinflammatory mediators in HS, may promote angiogenesis via the VEGFR-2 pathway, as well as comedogenesis in the follicular infundibulum (49, 50).
In acne vulgaris, follicular hyperproliferation and comedogenesis are induced by IL-1α, which is a potent angiogenic factor. Pattern recognition receptors, such as TLRs, recognize lipase, hyaluronidase and proteases released by C. acnes (51). In particular, the expression of TLR2 and TLR4 is increased in the epidermis of acne lesions, resulting in the production of IL-1, TNF-α, IL-8, prostaglandins, leukotrienes and MMP9 (52). In addition, C. acnes promotes the secretion of IL-1β and IL-18 through the inflammasome pathway via NLRP3 and caspase-1 (53). TH1 and TH17 cells in acne vulgaris also secrete proinflammatory cytokines, including interferon-γ, IL-1β TGF-β and IL-17. As such, inflammatory pathways shared with rosacea and HS contribute to angiogenesis in acne vulgaris.
Potential anti-angiogenic targets of metformin
Several attempts have been made to utilize metformin based on its anti-angiogenic effects. For example, recent in vivo studies of cancers showed that metformin inhibits angiogenesis by downregulating PDGF-B, hypoxia-inducible factor-1α (HIF-1α)-induced VEGF-A, IGFBP-2, IGFBP-3, PDGF-AA, MMP9, endostatin and angiogenin (54–56). PCOS is characterized by abnormal angiogenesis as well as insulin resistance. Metformin reduces angiogenesis in PCOS by raising the level of anti-angiogenic thrombospondin-1 (57). Several dermatological studies have investigated the therapeutic role of metformin in hirsutism, HS, acne in PCOS, psoriasis, acanthosis nigricans and skin cancers. Although its anti-angiogenic effects on rosacea, HS, and acne vulgaris remain unclear, the underlying mechanisms of action are expected to be similar to those in other diseases.
The specific mechanisms of the anti-angiogenic effects of metformin include the inhibition of VEGF expression and decreased microvessel density via the mTORC1 signalling pathway (58–60). Dysregulation of the mTORC1 signalling pathway, which regulates cellular growth, metabolism, and survival, may lead to uncontrolled proliferation and inflammation (61). The signalling pathway is activated in several inflammatory skin diseases, including atopic dermatitis, psoriasis, pemphigus, rosacea, HS and acne vulgaris (25, 61, 62). mTORC1 was shown to be activated in the endothelial cells of lesional skin derived from rosacea patients and an LL-37-induced rosacea-like mouse model (62). LL-37 activates mTORC1 signalling by binding to TLR2, which, in turn, increases the cleavage of cathelicidin to LL-37, in a positive feedback loop (63). The inhibition of mTORC1 signalling by rapamycin significantly reduced LL-37-induced vasculature in a mouse model (62). Other studies have reported that mTORC1 expression was increased in both the lesional and non-lesional skin of patients with HS and acne compared with normal controls (11, 25). Although the specific mechanism needs further investigation, considering that mTORC1 promotes TH17 cell differentiation (64), it may induce angiogenesis in HS and acne vulgaris in an IL-17-dependent manner.
In addition to the mTORC1 signalling pathway, metformin may exert its anti-angiogenic effects through other pathways. For instance, a mouse model of carotid artery atherosclerotic plaques showed that metformin was directly bound to MMP9, resulting in its degradation (65). MMP9 was reduced in a metformin-treated rosacea-like mouse model, and the possible binding of metformin and MMP9 was identified in molecular docking studies (66). Metformin also inhibited TNF-α, IL-1β and IL-17A-induced inflammatory responses by blocking the NLRP3 inflammasome in vitro (67). Since the overexpression of MMP9 and the activation of NLRP3 inflammasome are common in rosacea, HS and acne vulgaris, metformin also has the potential to reduce angiogenesis in those diseases. While further research is needed, it would be worth applying metformin to acne, rosacea, and HS patients as it targets angiogenic factors, such as mTORC1, VEGF, TLR2, IL-1, IL-17, NLRP3, and MMP9.
ANTI-FIBROTIC EFFECTS OF METFORMIN
Fibrosis in skin diseases
Fibrosis is a physiological response to injury and irritation, with the excessive deposition of connective tissue components in an organ (68). Excessive cutaneous fibrosis can lead to pathological conditions, including hypertrophic scars, keloids, chronic cutaneous graft-versus-host disease, and scleroderma (69). Over-deposition of ECM is attributed to abnormal ECM degradation and synthesis under chronic inflammatory conditions, such as hypertrophic scars, keloids, and rhinophyma seen in acne vulgaris, HS and rosacea.
ECM synthesis by activated fibroblasts and myofibroblasts is driven mainly by the TGF-β signalling pathway (69). In addition to TGF-β, oxidative stress, mechanical tension, and other profibrotic factors, such as connective tissue growth factor (CTGF), PDGF, FGF, IL-4, IL-5, IL-6, IL-13 and IL-21 promote ECM production (69, 70). TGF-β1 binds to its receptor and then phosphorylates the transcription factors Smad2/3, resulting in profibrotic gene expression (71).
Potential anti-fibrotic targets of metformin
Metformin decreases fibrosis by inhibiting TGF-β1 production, suppressing TGF-β from binding to its receptor, and blocking the phosphorylation of Smad3 (72–74). AMPK activation by metformin can inhibit the phosphorylation and nuclear translocation of Smad3 and decrease Smad3-mediated transcription (68). AMPK also induces the proteasomal degradation of P300, a coactivator of Smad2/3, which results in the reduced acetylation and transcription of Smad3 (75). The activation of AMPK also decreases TGF-β1-induced myofibroblast differentiation (76). Furthermore, the anti-fibrotic effects of metformin are mediated by the inhibition of HIF-1α-dependent fibroblast activation in hypoxic conditions and the downregulation of the PI3K-FoxO3 pathway (77, 78).
Metformin has been investigated as a potential treatment option for keloids based on its anti-fibrotic molecular mechanisms. Metformin decreased ECM components, such as collagen types I, Ⅲ, fibronectin, and elastin in keloid spheroids, an in vitro three-dimensional (3-D) keloid model (79). In another study, metformin inhibited the HIF-1α-dependent epithelial-to-mesenchymal transition of keloid fibroblasts (80). Efforts have also been made to generate a metformin-releasing multilayered 3-D scaffold to alleviate fibrosis and accelerate wound healing (81, 82). Further studies are needed to investigate the anti-fibrotic effects of metformin in acne vulgaris, HS and rosacea, which have shared pathways in fibrosis and are well-known to cause scars.
ANTIOXIDANT EFFECTS OF METFORMIN
Oxidative stress in skin diseases
Oxidative stress, a consequence of reactive oxygen species (ROS) production, plays a key role in the pathogenesis of various skin diseases and skin ageing. ROS are produced by various skin cells, including keratinocytes, in response to stimuli such as inflammatory cytokines, UV radiation, air pollutants, drugs, foods, and cosmetics (83, 84). They damage DNA, lipid membranes, proteins and other macromolecules inside cells. ROS also trigger signalling pathways related to activator protein 1 (AP-1), mitogen-activated protein kinase (MAPK), NF-κB and Akt (84). Antioxidants present in skin tissue inhibit this oxidative stress. Endogenous antioxidants include superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione S-transferase (GST), catalase, vitamin C, vitamin A, vitamin E, sulphydryl groups, glutathione, melatonin, carotenoids, flavonoids, coenzyme Q10 and selenium (83, 85).
However, in several studies, SOD and GPX activities were reduced in patients with acne compared with controls (86, 87). Linoleic acid suppresses the generation of ROS, but its level was found to be lower in patients with acne (88). Serum vitamin A and E levels were also lower in patients with acne (89, 90). Levels of ischaemia-modified albumin (IMA), which is formed under oxidative stress, were increased both in acne vulgaris and HS, and increases in IMA levels were proportional to disease severity (91, 92). Thiol, the antioxidant containing a sulphydryl group, is converted to disulphide under oxidative stress. The proportion of the disulphide form was higher in patients with HS compared with control groups (92), which suggests the possibility of higher oxidative stress in such patients. In rosacea, oxidative stress levels, represented by ferritin and plasma malondialdehyde (MDA) levels, were increased, whereas the antioxidant potential was decreased (93, 94). LL-37, which is a key factor in the pathogenesis of rosacea, stimulates the generation of ROS by activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and intracellular Ca2+ mobilization (95).
Potential antioxidant targets of metformin
As AMPK is a key regulator of ROS, metformin exhibits antioxidant effects by activating AMPK (96). In response to oxidative stress, AMPK facilitates antioxidant production by activating FoxO and inhibiting mTOR signalling (96). Metformin also decreases the production of ROS by inhibiting mitochondrial complex I (97). It may potentiate the antioxidant effect via enhanced autophagy, which allows cellular adaptation to oxidative stress (98, 99). Furthermore, metformin reduces MDA levels and upregulates the expression of antioxidant enzymes, such as GST and SOD (100–102). In this regard, metformin has potential therapeutic benefits for patients with acne vulgaris, HS and rosacea, conditions that are associated with increased oxidative stress.
CLINICAL STUDIES OF METFORMIN INVOLVING ACNE VULGARIS, HIDRADENITIS SUPPURATIVA AND ROSACEA
Acne vulgaris
Studies investigating metformin as a treatment option for acne have mostly been conducted in patients with PCOS. In a systematic review and meta-analysis (103) of 27 randomized controlled trials, 5 non-randomized controlled trials, and 19 open-label studies involving patients with PCOS, metformin adjuvant therapy raised the acne scores more than the regimen without metformin (standardized mean difference (SMD) –0.256; 95% confidence interval (95% CI) –0.439 to –0.074). Acne scores decreased significantly after metformin treatment (SMD –0.712; 95% CI –0.949 to –0.476). However, oral contraceptive pills were superior to metformin in treating acne vulgaris in patients with PCOS, according to a Cochrane review (104), which compared the effectiveness and safety of metformin vs oral contraceptive pills in women with PCOS.
Few clinical studies have investigated the therapeutic role of metformin in patients with acne without PCOS (105–107). A randomized open-label study (105) investigated the effectiveness of metformin as adjuvant therapy for moderate-to-severe facial acne in patients, regardless of sex, body mass index (BMI) or insulin resistance. Eighty-four patients were treated with either oral tetracycline at 250 mg twice daily combined with 2.5% topical benzoyl peroxide with or without metformin at 850 mg daily. The clinical severity of acne was assessed at weeks 0, 6 and 12, based on the total number of acne lesions, Investigator’s Global Assessment (IGA) scores, and Cardiff Acne Disability Index (CADI) scores. Patients who received metformin had a higher treatment success rate (p = 0.04), a greater mean percentage reduction from baseline in total lesion counts (p = 0.278), and a greater mean reduction in CADI scores (p = 0.451) than those without metformin. The study also revealed that metformin adjuvant therapy significantly improved the clinical severity of acne, irrespective of BMI status.
Another randomized controlled study (106) involved 20 male patients with an altered metabolic profile (defined as impaired fasting glucose, increased levels of total and low-density lipoprotein cholesterol, decreased levels of high-density lipoprotein cholesterol, and waist circumference and BMI at the upper limits of normal). An experimental group of 10 patients who were treated with metformin and a hypocaloric diet for 6 months had statistically significant improvements in acne compared with the control group (p < 0.05).
A recent observational study (107) assessed the effectiveness of metformin alone in acne vulgaris. Thirty patients were treated with metformin at 1,000 mg daily for 3 months without any other topical or systemic therapy for acne. Metformin monotherapy significantly improved the clinical severity of acne based on global acne grading scores (p < 0.001).
Hidradenitis suppurativa
In 1 prospective cohort study (108), 25 patients with HS previously refractory to standard therapies were treated with metformin over a period of 24 weeks. The clinical severity of HS was assessed at weeks 0, 12 and 24, based on both Sartorius and Dermatology Life Quality Index (DLQI) scores. The results showed clinical improvement in 72% (n = 18/25), with a mean reduction in Sartorius scores of 12.7. The DLQI score also improved in 76% of the patients (n = 19/25), with a mean reduction of 7.6.
In a retrospective analysis of 53 patients with HS treated with metformin (109), a subjective clinical response was observed in 68% (n = 36/53), of which 19% (n = 7/36) showed a complete response (no active HS lesions reviewed by a dermatologist) to metformin monotherapy. The mean treatment duration was 11.3 months, and the mean dose was 1.5 g/day. The analysis showed no correlation between insulin resistance and the clinical response to metformin.
Another retrospective analysis was conducted in 16 paediatric patients with HS treated with metformin as adjunctive therapy (110). Clinical improvement was observed in 31% of the patients (n = 5/16), with 38% (n = 6/16) lost to follow-up. Two patients discontinued metformin due to gastrointestinal distress and mood changes.
Rosacea
Despite studies at the cellular and animal levels, no clinical studies have investigated the effect of metformin on rosacea in humans.
Conclusion
Hyperandrogenism, angiogenesis, fibrosis and oxidative stress contribute to the outbreak, aggravation and sequelae of acne vulgaris, HS and rosacea. Metformin, a widely used hypoglycaemic drug with proven safety, has multiple beneficial effects besides lowering serum glucose. Based on its anti-androgenic, anti-angiogenic, anti-fibrotic and antioxidant effects, metformin represents a novel therapeutic candidate for acne vulgaris, HS and rosacea. The possible therapeutic targets of metformin in each disease are summarized in Figs 1–3. Further laboratory and clinical investigations are required to elucidate the therapeutic mechanisms and clinical efficacy of metformin in dermatological diseases.
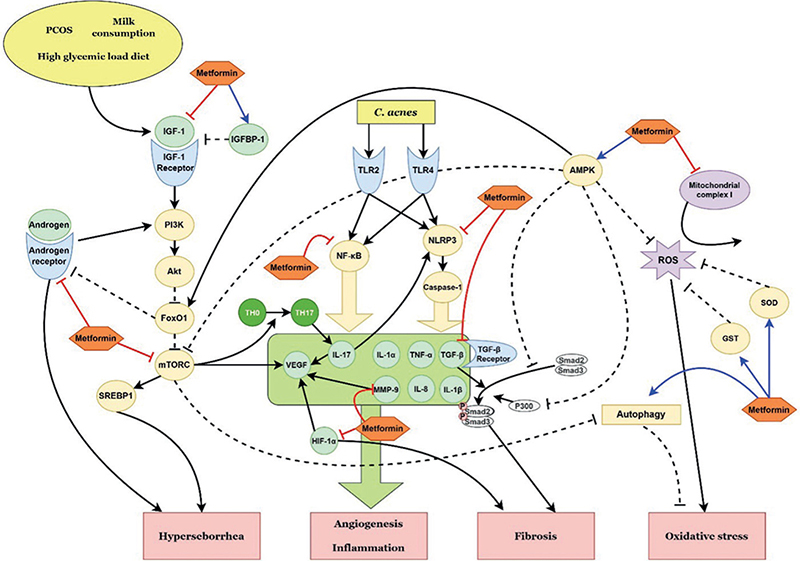
Fig. 1. Schematic representation of the possible therapeutic targets of metformin in acne vulgaris, according to the pathways identified in this review. PCOS: polycystic ovary syndrome; IGF-1: insulin-like growth factor-1; IGFBP-1: insulin-like growth factor-binding protein-1; PI3K: phosphoinositol-3-kinase; FoxO1: forkhead box-O1; mTORC: mammalian target of rapamycin complex; SREBP: sterol regulatory element binding protein; C. acnes: Cutibacterium acnes; TLR: Toll-like receptor; NF-κB: nuclear factor kappa B; NLRP3: NLR family pyrin domain containing 3; IL: interleukin; TH0: naïve T helper cell; TH17: T helper type 17 cell; VEGF: vascular endothelial growth factor; MMP: matrix metalloproteinase; TNF-α: tumour necrosis factor-α; TGF-β: transforming growth factor-β; HIF-1α: hypoxia-inducible factor-1α; AMPK: adenosine monophosphate-activated protein kinase; ROS: reactive oxygen species; SOD: superoxide dismutase; GST: glutathione S-transferase.
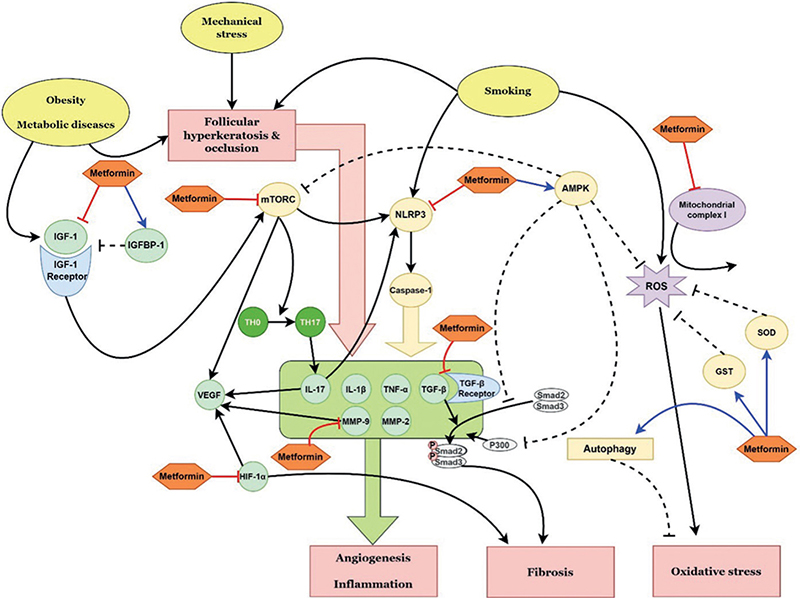
Fig. 2. Schematic representation of the possible therapeutic targets of metformin in hidradenitis suppurativa, according to the pathways identified in this review. IGF-1: insulin-like growth factor-1; IGFBP-1: insulin-like growth factor-binding protein-1; mTORC: mammalian target of rapamycin complex; NLRP3: NLR family pyrin domain containing 3; IL: interleukin; TH0: naïve T helper cell; TH17: T helper type 17 cell; VEGF: vascular endothelial growth factor; MMP: matrix metalloproteinase; TNF-α: tumour necrosis factor-α; TGF-β: transforming growth factor-β; HIF-1α: hypoxia-inducible factor-1α; AMPK: adenosine monophosphate-activated protein kinase; ROS: reactive oxygen species; SOD: superoxide dismutase; GST: glutathione S-transferase.
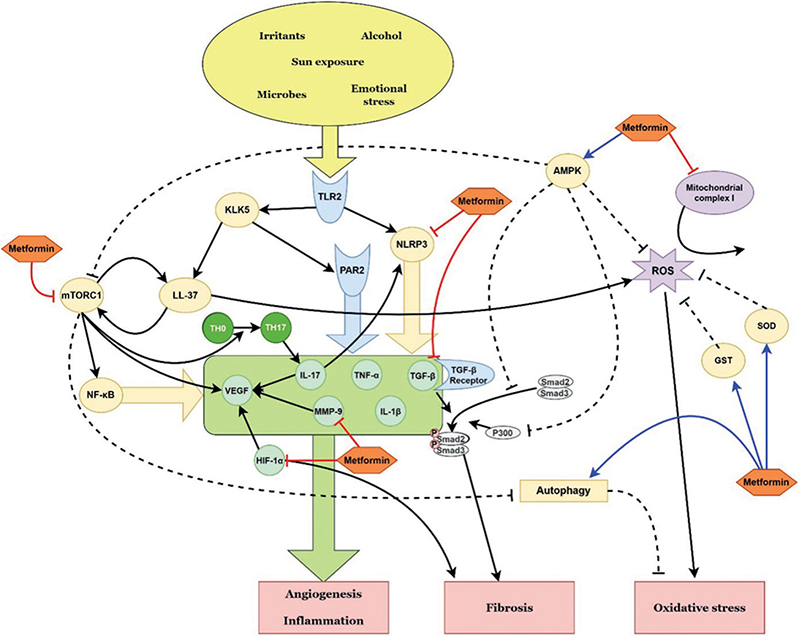
Fig. 3. Schematic representation of the possible therapeutic targets of metformin in rosacea, according to the pathways identified in this review. TLR: Toll-like receptor; KLK5: kallikrein 5; PAR2: protease-activated receptor 2; mTORC1: mammalian target of rapamycin complex 1; NLRP3: NLR family pyrin domain containing 3; IL: interleukin; TH0: naïve T helper cell; TH17: T helper type 17 cell; VEGF: vascular endothelial growth factor; MMP: matrix metalloproteinase; TNF-α: tumour necrosis factor-α; TGF-β: transforming growth factor-β; HIF-1α: hypoxia-inducible factor-1α; AMPK: adenosine monophosphate-activated protein kinase; ROS: reactive oxygen species; SOD: superoxide dismutase; GST: glutathione S-transferase.
ACKNOWLEDGEMENT
This work was supported by the Po-Ca Networking Groups funded by The Postech-Catholic Biomedical Engineering Institute(PCBMI) (No. 5-2023-B0001-00034).
REFERENCES
- Nasri H, Rafieian-Kopaei M. Metformin: current knowledge. J Res Med Sci 2014; 19: 658–664.
- Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol (Lausanne) 2020; 11: 191.
- Badr D, Kurban M, Abbas O. Metformin in dermatology: an overview. J Eur Acad Dermatol Venereol 2013; 27: 1329–1335.
- Bubna AK. Metformin – for the dermatologist. Ind J Pharmacol 2016; 48: 4–10.
- Podhorecka M, Ibanez B, Dmoszyńska A. Metformin – its potential anti-cancer and anti-aging effects. Postepy Hig Med Dosw (Online) 2017; 71: 170–175.
- Bienenfeld A, Azarchi S, Lo Sicco K, Marchbein S, Shapiro J, Nagler AR. Androgens in women: Androgen-mediated skin disease and patient evaluation. J Am Acad Dermatol 2019; 80: 1497–1506.
- Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res 2012; 304: 499–510.
- Ceruti JM, Leirós GJ, Balañá ME. Androgens and androgen receptor action in skin and hair follicles. Mol Cell Endocrinol 2018; 465: 122–133.
- Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol 2004; 22: 360–366.
- Mirdamadi Y, Bommhardt U, Goihl A, Guttek K, Zouboulis CC, Quist S, et al. Insulin and Insulin-like growth factor-1 can activate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in T cells in vitro. Dermatoendocrinol 2017; 9: e1356518.
- Monfrecola G, Lembo S, Caiazzo G, De Vita V, Di Caprio R, Balato A, et al. Mechanistic target of rapamycin (mTOR) expression is increased in acne patients’ skin. Exp Dermatol 2016; 25: 153–155.
- Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol 2004; 123: 1–12.
- Mirdamadi Y, Thielitz A, Wiede A, Goihl A, Papakonstantinou E, Hartig R, et al. Insulin and insulin-like growth factor-1 can modulate the phosphoinositide-3-kinase/Akt/FoxO1 pathway in SZ95 sebocytes in vitro. Mol Cell Endocrinol 2015; 415: 32–44.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol 2009; 18: 833–841.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2020; 82: 1045–1058.
- Harrison BJ, Kumar S, Read GF, Edwards CA, Scanlon MF, Hughes LE. Hidradenitis suppurativa: evidence for an endocrine abnormality. Br J Surg 1985; 72: 1002–1004.
- Mortimer PS, Dawber RP, Gales MA, Moore RA. Mediation of hidradenitis suppurativa by androgens. Br Med J (Clin Res Ed) 1986; 292: 245–248.
- Margesson LJ, Danby FW. Hidradenitis suppurativa. Best Pract Res Clin Obstet Gynaecol 2014; 28: 1013–1027.
- Barth JH, Kealey T. Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa. Br J Dermatol 1991; 125: 304–308.
- Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg 2007; 11: 125–131.
- Goldsmith PC, Dowd PM. Successful therapy of the follicular occlusion triad in a young woman with high dose oral antiandrogens and minocycline. J R Soc Med 1993; 86: 729–730.
- Mortimer PS, Dawber RP, Gales MA, Moore RA. A double-blind controlled cross-over trial of cyproterone acetate in females with hidradenitis suppurativa. Br J Dermatol 1986; 115: 263–268.
- Golbari NM, Porter ML, Kimball AB. Antiandrogen therapy with spironolactone for the treatment of hidradenitis suppurativa. J Am Acad Dermatol 2019; 80: 114–119.
- Babbush KM, Andriano TM, Cohen SR. Antiandrogen therapy in hidradenitis suppurativa: finasteride for females. Clin Exp Dermatol 2022; 47: 86–92.
- Monfrecola G, Balato A, Caiazzo G, De Vita V, Di Caprio R, Donnarumma M, et al. Mammalian target of rapamycin, insulin resistance and hidradenitis suppurativa: a possible metabolic loop. J Eur Acad Dermatol Venereol 2016; 30: 1631–1633.
- Herman R, Kravos NA, Jensterle M, Janež A, Dolžan V. Metformin and insulin resistance: a review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int J Mol Sci 2022; 23: 1264.
- Chen K, Li Y, Guo Z, Zeng Y, Zhang W, Wang H. Metformin: current clinical applications in nondiabetic patients with cancer. Aging (Albany NY) 2020; 12: 3993–4009.
- Pawelczyk L, Spaczynski RZ, Banaszewska B, Duleba AJ. Metformin therapy increases insulin-like growth factor binding protein-1 in hyperinsulinemic women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 2004; 113: 209–213.
- Hu M, Zhang Y, Li X, Cui P, Sferruzzi-Perri AN, Brännström M, et al. TLR4-associated IRF-7 and NFκB signaling act as a molecular link between androgen and metformin activities and cytokine synthesis in the PCOS endometrium. J Clin Endocrinol Metab 2021; 106: 1022–1040.
- Ohara M, Yoshida-Komiya H, Ono-Okutsu M, Yamaguchi-Ito A, Takahashi T, Fujimori K. Metformin reduces androgen receptor and upregulates homeobox A10 expression in uterine endometrium in women with polycystic ovary syndrome. Reprod Biol Endocrinol 2021; 19: 77.
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–1186
- Fried LE, Arbiser JL. Application of angiogenesis to clinical dermatology. Adv Dermatol 2008; 24: 89–103.
- Barnhill RL, Wolf JE, Jr. Angiogenesis and the skin. J Am Acad Dermatol 1987; 16: 1226–1242.
- Lee HJ, Hong YJ, Kim M. Angiogenesis in chronic inflammatory skin disorders. Int J Mol Sci 2021; 22: 12035.
- Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis 2007; 39: 212–220.
- Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol 2002; 282: C947–C970.
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011; 473: 298–307.
- Goumans MJ, Ten Dijke P. TGF-β signaling in control of cardiovascular function. Cold Spring Harb Perspect Biol 2018; 10: a022210.
- Varricchi G, Granata F, Loffredo S, Genovese A, Marone G. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol 2015; 73: 144–153.
- Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med 2007; 13: 975–980.
- Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol 2011; 131: 688–697.
- Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, et al. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 2012; 7: e29695.
- Oikonomopoulou K, Hansen KK, Saifeddine M, Vergnolle N, Tea I, Blaber M, et al. Kallikrein-mediated cell signalling: targeting proteinase-activated receptors (PARs). Biol Chem 2006; 387: 817–824.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res 2018; 7: F1000 Faculty Rev-1885.
- Gomaa AH, Yaar M, Eyada MM, Bhawan J. Lymphangiogenesis and angiogenesis in non-phymatous rosacea. J Cutan Pathol 2007; 34: 748–753.
- Shah A, Alhusayen R, Amini-Nik S. The critical role of macrophages in the pathogenesis of hidradenitis suppurativa. Inflamm Res 2017; 66: 931–945.
- Vilanova I, Hernández JL, Mata C, Durán C, García-Unzueta MT, Portilla V, et al. Insulin resistance in hidradenitis suppurativa: a case-control study. J Eur Acad Dermatol Venereol 2018; 32: 820–824.
- Napolitano M, Megna M, Monfrecola G. Insulin resistance and skin diseases. ScientificWorldJournal 2015; 2015: 479354.
- Frew JW. Hidradenitis suppurativa is an autoinflammatory keratinization disease: A review of the clinical, histologic, and molecular evidence. JAAD Int 2020; 1: 62–72.
- Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1alpha promotes angiogenesis in vivo via VEGFR-2 pathway by inducing inflammatory cell VEGF synthesis and secretion. Faseb J 2002; 16: 1471–1473.
- Firlej E, Kowalska W, Szymaszek K, Roliński J, Bartosińska J. The role of skin immune system in acne. J Clin Med 2022; 11: 1579.
- Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol 2005; 153: 1105–1113.
- Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE, Lee Y, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol 2014; 134: 2747–2756.
- Wang JC, Li GY, Wang B, Han SX, Sun X, Jiang YN, et al. Metformin inhibits metastatic breast cancer progression and improves chemosensitivity by inducing vessel normalization via PDGF-B downregulation. J Exp Clin Cancer Res 2019; 38: 235.
- Wang J, Li G, Wang Y, Tang S, Sun X, Feng X, et al. Suppression of tumor angiogenesis by metformin treatment via a mechanism linked to targeting of HER2/HIF-1α/VEGF secretion axis. Oncotarget 2015; 6: 44579–44592.
- Orecchioni S, Reggiani F, Talarico G, Mancuso P, Calleri A, Gregato G, et al. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer 2015; 136: E534–E544.
- Tan BK, Adya R, Chen J, Farhatullah S, Heutling D, Mitchell D, et al. Metformin decreases angiogenesis via NF-kappaB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res 2009; 83: 566–574.
- Tadakawa M, Takeda T, Li B, Tsuiji K, Yaegashi N. The anti-diabetic drug metformin inhibits vascular endothelial growth factor expression via the mammalian target of rapamycin complex 1/hypoxia-inducible factor-1α signaling pathway in ELT-3 cells. Mol Cell Endocrinol 2015; 399: 1–8.
- Liao H, Zhou Q, Gu Y, Duan T, Feng Y. Luteinizing hormone facilitates angiogenesis in ovarian epithelial tumor cells and metformin inhibits the effect through the mTOR signaling pathway. Oncol Rep 2012; 27: 1873–1878.
- Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia 2011; 13: 483–491.
- Karagianni F, Pavlidis A, Malakou LS, Piperi C, Papadavid E. Predominant role of mTOR signaling in skin diseases with therapeutic potential. Int J Mol Sci 2022; 23: 1693.
- Peng Q, Sha K, Liu Y, Chen M, Xu S, Xie H, et al. mTORC1-mediated angiogenesis is required for the development of rosacea. Front Cell Dev Biol 2021; 9: 751785.
- Deng Z, Chen M, Liu Y, Xu S, Ouyang Y, Shi W, et al. A positive feedback loop between mTORC1 and cathelicidin promotes skin inflammation in rosacea. EMBO Mol Med 2021; 13: e13560.
- Nagai S, Kurebayashi Y, Koyasu S. Role of PI3K/Akt and mTOR complexes in Th17 cell differentiation. Ann N Y Acad Sci 2013; 1280: 30–34.
- Chen X, Wang S, Xu W, Zhao M, Zhang Y, Xiao H. Metformin directly binds to MMP-9 to improve plaque stability. J Cardiovasc Dev Dis 2023; 10: 54.
- Li Y, Yang L, Wang Y, Deng Z, Xu S, Xie H, et al. Exploring metformin as a candidate drug for rosacea through network pharmacology and experimental validation. Pharmacol Res 2021; 174: 105971.
- Tsuji G, Hashimoto-Hachiya A, Yen VH, Takemura M, Yumine A, Furue K, et al. Metformin inhibits IL-1β secretion via impairment of NLRP3 inflammasome in keratinocytes: implications for preventing the development of psoriasis. Cell Death Discov 2020; 6: 11.
- Wu M, Xu H, Liu J, Tan X, Wan S, Guo M, et al. Metformin and fibrosis: a review of existing evidence and mechanisms. J Diabetes Res 2021; 2021: 6673525.
- Do NN, Eming SA. Skin fibrosis: models and mechanisms. Curr Res Transl Med 2016; 64: 185–193.
- Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol 2015; 47: 54–65.
- Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact 2018; 292: 76–83.
- Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, et al. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res 2010; 87: 504–513.
- Yi H, Huang C, Shi Y, Cao Q, Zhao Y, Zhang L, et al. Metformin attenuates folic-acid induced renal fibrosis in mice. J Cell Physiol 2018; 233: 7045–7054.
- Xiao H, Zhang J, Xu Z, Feng Y, Zhang M, Liu J, et al. Metformin is a novel suppressor for transforming growth factor (TGF)-β1. Sci Rep 2016; 6: 28597.
- Lim JY, Oh MA, Kim WH, Sohn HY, Park SI. AMP-activated protein kinase inhibits TGF-β-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J Cell Physiol 2012; 227: 1081–1089.
- Cheng D, Xu Q, Wang Y, Li G, Sun W, Ma D, et al. Metformin attenuates silica-induced pulmonary fibrosis via AMPK signaling. J Transl Med 2021; 19: 349.
- Biller ML, Tuffs C, Bleul M, Tran DT, Dupovac M, Keppler U, et al. Effect of metformin on HIF-1α signaling and postoperative adhesion formation. J Am Coll Surg 2022; 234: 1167–1180.
- Kim JM, Yoo H, Kim JY, Oh SH, Kang JW, Yoo BR, et al. Metformin alleviates radiation-induced skin fibrosis via the downregulation of FOXO3. Cell Physiol Biochem 2018; 48: 959–970.
- Jeon HB, Roh H, Ahn HM, Lee JH, Yun CO, Roh TS, et al. Metformin inhibits transforming growth factor β-induced fibrogenic response of human dermal fibroblasts and suppresses fibrosis in keloid spheroids. Ann Plast Surg 2021; 86: 406–411.
- Lei R, Zhang S, Wang Y, Dai S, Sun J, Zhu C. Metformin inhibits epithelial-to-mesenchymal transition of keloid fibroblasts via the HIF-1α/PKM2 signaling pathway. Int J Med Sci 2019; 16: 960–966.
- Harmanci S, Dutta A, Cesur S, Sahin A, Gunduz O, Kalaskar DM, et al. Production of 3D printed bi-layer and tri-layer sandwich scaffolds with polycaprolactone and poly (vinyl alcohol)-metformin towards diabetic wound healing. Polymers (Basel) 2022; 14: 5306.
- Chogan F, Mirmajidi T, Rezayan AH, Sharifi AM, Ghahary A, Nourmohammadi J, et al. Design, fabrication, and optimization of a dual function three-layer scaffold for controlled release of metformin hydrochloride to alleviate fibrosis and accelerate wound healing. Acta Biomater 2020; 113: 144–163.
- Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep 2016; 21: 164–169.
- Nakai K, Tsuruta D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases?. Int J Mol Sci 2021; 22: 10799.
- Addor FAS. Antioxidants in dermatology. An Bras Dermatol 2017; 92: 356–362.
- Basak PY, Gultekin F, Kilinc I. The role of the antioxidative defense system in papulopustular acne. J Dermatol 2001; 28: 123–127.
- Kurutas EB, Arican O, Sasmaz S. Superoxide dismutase and myeloperoxidase activities in polymorphonuclear leukocytes in acne vulgaris. Acta Dermatovenerol Alp Pannonica Adriat 2005; 14: 39–42.
- Akamatsu H, Horio T. The possible role of reactive oxygen species generated by neutrophils in mediating acne inflammation. Dermatology 1998; 196: 82–85.
- Ozuguz P, Dogruk Kacar S, Ekiz O, Takci Z, Balta I, Kalkan G. Evaluation of serum vitamins A and E and zinc levels according to the severity of acne vulgaris. Cutan Ocul Toxicol 2014; 33: 99–102.
- El-Akawi Z, Abdel-Latif N, Abdul-Razzak K. Does the plasma level of vitamins A and E affect acne condition? Clin Exp Dermatol 2006; 31: 430–434.
- Tunçez Akyürek F, Saylam Kurtipek G, Kurku H, Akyurek F, Unlu A, Abusoglu S, et al. Assessment of ADMA, IMA, and vitamin A and E levels in patients with acne vulgaris. J Cosmet Dermatol 2020; 19: 3408–3413.
- Balik ZB, Balik AR, Yucel C, Hayran Y, Çaliskan E, Erel O. Investigation of thiol-disulfide homeostasis and ischemia-modified albumin levels in patients with hidradenitis supurativa. J Cosmet Dermatol 2022; 21: 4748–4753.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, Brcic L, Dobric I, Lipozencic J, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol 2009; 60: 270–276.
- Baz K, Cimen MY, Kokturk A, Aslan G, Ikizoglu G, Demirseren DD, et al. Plasma reactive oxygen species activity and antioxidant potential levels in rosacea patients: correlation with seropositivity to Helicobacter pylori. Int J Dermatol 2004; 43: 494–497.
- Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol 2007; 157: 1124–1131.
- Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer 2017; 16: 79.
- Kelly B, Tannahill GM, Murphy MP, O’Neill LA. Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem 2015; 290: 20348–20359.
- Ren H, Shao Y, Wu C, Ma X, Lv C, Wang Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol 2020; 500: 110628.
- Packer M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc Diabetol 2020; 19: 62.
- Araújo AA, Pereira A, Medeiros C, Brito GAC, Leitão RFC, Araújo LS, et al. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PLoS One 2017; 12: e0183506.
- Sharma P, Kumar S. Metformin inhibits human breast cancer cell growth by promoting apoptosis via a ROS-independent pathway involving mitochondrial dysfunction: pivotal role of superoxide dismutase (SOD). Cell Oncol (Dordr) 2018; 41: 637–650.
- Tripathi SS, Singh S, Garg G, Kumar R, Verma AK, Singh AK, et al. Metformin ameliorates acetaminophen-induced sub-acute toxicity via antioxidant property. Drug Chem Toxicol 2022; 45: 52–60.
- Yen H, Chang YT, Yee FJ, Huang YC. Metformin therapy for acne in patients with polycystic ovary syndrome: a systematic review and meta-analysis. Am J Clin Dermatol 2021; 22: 11–23.
- Fraison E, Kostova E, Moran LJ, Bilal S, Ee CC, Venetis C, et al. Metformin versus the combined oral contraceptive pill for hirsutism, acne, and menstrual pattern in polycystic ovary syndrome. Cochrane Database Syst Rev 2020; 8: Cd005552.
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study. Dermatol Ther 2019; 32: e12953.
- Fabbrocini G, Izzo R, Faggiano A, Del Prete M, Donnarumma M, Marasca C, et al. Low glycaemic diet and metformin therapy: a new approach in male subjects with acne resistant to common treatments. Clin Exp Dermatol 2016; 41: 38–42.
- Kamboj P, Kaushik A, Handa S, Dutta P, Saikia UN, Pal A, et al. Effects of metformin on clinical, hormonal and relevant gene expression parameters in patients of acne – an observational study. Clin Exp Dermatol 2023; 48: 617–622.
- Verdolini R, Clayton N, Smith A, Alwash N, Mannello B. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol 2013; 27: 1101–1108.
- Jennings L, Hambly R, Hughes R, Moriarty B, Kirby B. Metformin use in hidradenitis suppurativa. J Dermatolog Treat 2020; 31: 261–263.
- Moussa C, Wadowski L, Price H, Mirea L, O’Haver J. Metformin as adjunctive therapy for pediatric patients with hidradenitis suppurativa. J Drugs Dermatol 2020; 19: 1231–1234.