REVIEW ARTICLE
Effect of Laser and Energy-based Device Therapies to Minimize Surgical Scar Formation: A Systematic Review and Network Meta-analysis
Sasitorn YENYUWADEE1, Petchlada ACHAVANUNTAKUL2, Pochamana PHISALPRAPA3, Melissa K. LEVIN4, Surasak SAOKAEW5–10, Sukrit KANCHANASURAKIT6–8, 11, 12 and Woraphong MANUSKIATTI1
1Departments of Dermatology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, 2Department of Medicine, College of Medicine and Public Health, Ubonratchathani University, Ubonratchathani, 3Division of Ambulatory Medicine, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, 4Entière Dermatology, New York, USA, 5Division of Social and Administration Pharmacy, Department of Pharmaceutical Care, 6Center of Health Outcomes Research and Therapeutic Safety (Cohorts), 7Unit of Excellence on Clinical Outcomes Research and Integration (UNICORN), 8Unit of Excellence on Herbal Medicine, School of Pharmaceutical Sciences, University of Phayao, Phayao, Thailand, 9Novel Bacteria and Drug Discovery Research Group, Microbiome and Bioresource Research Strength, Jeffrey Cheah School of Medicine and Health Sciences, 10Biofunctional Molecule Exploratory Research Group, Biomedicine Research Advancement Centre, School of Pharmacy, Monash University Malaysia, Bander Sunway, Malaysia, 11Division of Clinical Pharmacy, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao, and 12Division of Pharmaceutical Care, Department of Pharmacy, Phrae Hospital, Phrae, Thailand
Utilization of lasers and energy-based devices for surgical scar minimization has been substantially evaluated in placebo-controlled trials. The aim of this study was to compare reported measures of efficacy of lasers and energy-based devices in clinical trials in preventing surgical scar formation in a systematic review and network meta-analyses. Five electronic databases, PubMed, Scopus, Embase, ClinicalTrials.gov, and the Cochrane Library, were searched to retrieve relevant articles. The search was limited to randomized controlled trials that reported on clinical outcomes of surgical scars with treatment initiation no later than 6 months after surgery and a follow-up period of at least 3 months. A total of 18 randomized controlled trials involving 482 participants and 671 postsurgical wounds were included in the network meta-analyses. The results showed that the most efficacious treatments were achieved using low-level laser therapy) (weighted mean difference –3.78; 95% confidence interval (95% CI) –6.32, –1.24) and pulsed dye laser (weighted mean difference –2.46; 95% CI –4.53, –0.38). Nevertheless, low-level laser therapy and pulsed dye laser demonstrated comparable outcomes in surgical scar minimization (weighted mean difference –1.32, 95% CI –3.53, 0.89). The findings of this network meta-analyses suggest that low-level laser therapy and pulsed dye laser are both effective treatments for minimization of scar formation following primary closure of surgical wounds with comparable treatment outcomes.
Key words: surgical scar; laser; energy-based device; prevention; minimization; mitigation.
SIGNIFICANCE
Various types of lasers and energy-based devices have been used to prevent scar formation following primary closure of surgical wounds. Although data are promising, they are insufficient to draw firm conclusions about the relative efficacies of these treatments. In this systematic review and network meta-analysis of 18 randomized clinical trials including 482 participants and 671 postsurgical scars, low-level laser and pulsed dye laser treatment were associated with significant reductions in Vancouver Scar Scale score than the untreated control. This network meta-analysis may help clinicians and patients to make informed treatment choices for the prophylaxis of surgical scar formation.
Citation: Acta Derm Venereol 2024; 104: adv18477. DOI https://doi.org/10.2340/actadv.v104.18477.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Aug 23, 2023; Accepted: Nov 17, 2023; Published: Jan 8, 2024
Corr: Sukrit Kanchanasurakit, Division of Clinical Pharmacy, Department of Pharmaceutical Care, School of Pharmaceutical Sciences, University of Phayao, Phayao 56000, Thailand and Woraphong Manuskiatti, Department of Dermatology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand. E-mails: sukrit.ka@up.ac.th; woraphong.man@mahidol.edu
INTRODUCTION
Wound healing is a complex tissue-response to injury that leads to skin restoration. Cutaneous aberrant scarring is characterized by an imbalance between cell growth and excessive deposition of extracellular matrix and is the consequence of skin injury. Alterations in the wound healing process can result in hypertrophic or keloid scarring causing functional impairment and psychological distress, especially when the scars are on a conspicuous part of the body (1). Therefore, effective interventions to minimize postoperative and/or posttraumatic scar formation are essential.
While lasers and energy-based devices (EBDs) have been generally accepted as a prophylaxis against scarring, there is no consensus on the appropriate timing for initiation of treatment for best outcomes. Laser treatments for scars have traditionally been performed at a minimum of 2–3 months after surgery because of scar stabilization and the disappearance of erythema at the operation site (2). However, increasing evidence emphasizes the importance of earlier initiation of treatment in scar minimization (3–5). The sparsity of head-to-head trials for scar minimization treatment modalities makes direct comparisons of their efficacy difficult. Few meta-analyses have been conducted, with 1 meta-analysis demonstrating that laser treatments result in scar prevention (4). A systematic review and a network meta-analysis (NMA) were conducted to assess and compare the efficacy of laser and EBD therapies in patients who had undergone intervention for scar prevention after primary closure of surgical wounds.
MATERIALS AND METHODS
Protocol and registration
A systematic review and NMA were performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for NMA (6). This study was registered with the trial registration number CRD42022381360 under the international prospective register of systematic reviews (PROSPERO: www.crd.york.ac.uk/PROSPERO).
Eligibility and exclusion criteria for considering studies for this review
The analysis included randomized controlled trials (RCTs) that treated participants with lasers, EBDs or silicone gels and compared them with conventional postsurgical wound care to prevent postoperative scars. The primary outcome was differences in the mean Vancouver Scar Scale (VSS) score between the baseline and at the latest follow-up time-point. The secondary outcome was the mean difference of Observer Scar Assessment Scale (OSAS) between baseline and the latest follow-up visit. The study protocol only included interventions that were performed within 6 months after surgery and those with follow-up periods of more than, or equal to, 3 months after primary closure of the wounds in order to evaluate their effects on surgical scarring. RCTs that lacked sufficient information, such as standard deviation (SD), or studies that did not perform RCTs were not included in the current analysis.
Information sources and search strategy
Searches were performed for relevant published articles from 5 electronic databases (PubMed, Scopus, Embase, ClinicalTrials.gov, and the Cochrane Library) up until 24 January 2023, and medical subject headings (MeSH) were applied to each database, as applicable. The keywords included “pulsed dye laser”, “fractional laser”, “Nd:YAG laser”, “picosecond laser”, “erbium:YAG (Er:YAG) laser”, “diode laser”, “KTP laser”, “fractional carbon dioxide (CO2) laser”, “fractional erbium glass (Er:glass) laser”, “fractional erbium YAG (Er:YAG) laser”, “intense pulsed light (IPL)”, “low-level light”, “radiofrequency device”, “surgical wound”, “surgical scar”. Bibliographic lists of related articles were also explored. The complete search strategy is provided in Appendix S1; Table SI.
Study selection
Two investigators (SY and PA) independently evaluated the titles and abstracts, to identify potential eligibility from the searches, and relevant full-text articles were retrieved. The full-text articles were then assessed for final eligibility by the same individuals. Only English articles were included in the evaluation. Conflicts arising from the full-text articles were resolved through discussion or consultation with a team of experts.
Data extraction and study appraisal
The 2 investigators evaluated all potentially pertinent papers in a full-text search against the qualifying standards before selecting the paper in a data-extraction procedure. Data were extracted, including the location of study, study design, intervention details (such as the regimen, treatment parameter, and duration of treatment), the study size (number of patients and number of scars); population characteristics (age, Fitzpatrick skin type, anatomical area of the scar) and treatment outcomes (i.e. the reported mean and/or standard deviation (SD) values of VSS score and OSAS score at the last follow-up), which were the indicative measures of the effects of the interventions. When mean and/or SD were not reported, continuous outcomes were estimated by using the reported statistics (e.g. median, interquartile range, etc.) (7). Study authors were contacted to obtain the missing outcomes of the relevant studies. If the authors did not respond within 1 month, the study was excluded from the analysis.
Quality assessments
Using the updated Cochrane risk-of-bias tool for randomized trials (RoB 2.0, London, UK), 2 researchers independently evaluated each study’s risk of bias (8). This assessment addressed specific bias domains, including methods for generating the random sequence; allocation concealment; blinding of participants and investigators; blinding of the outcome assessment; incompleteness of the outcome data; and selective outcome reporting. The outcomes of each study’s assessment of each item were displayed in the risk-of-bias summary graph and the risk-of-bias summary itself. Adjudication of the risk of bias was completed by answering pre-specified questions concerning the methodologies reported by each study in relation to the risk domain.The results were either a low risk of bias, an uncertain risk of bias, or a high risk of bias. Any differences between the 2 researchers were settled through consensus or by consulting an expert.
Synthesis and statistical analysis
Pairwise meta-analyses were performed using the DerSimonian and Laird random effects model to estimate outcomes (9). The outcomes were then reported as weighted mean differences and 95% confidence intervals (95% CIs). The I-squared statistic and the χ2 test were used to evaluate the statistical heterogeneity in each pairwise comparison. When the p-value was less than 0.1, heterogeneity was considered to exist. Also, we used the network command in Stata Statistical Software: Version 16 (StataCorp LP, College Station, TX, USA) and the random-effects NMA techniques outlined by Lu and Ades to integrate direct and indirect evidence of all relative alternative effects (10). To rank the options, hierarchy of competing for intervention in the NMA, the rankogram; the surface under the cumulative ranking (SUCRA) curves; the mean ranks; and the league tables were used (11). A global inconsistency test was used to evaluate the network consistency between direct and indirect evidence (p-value ≥ 0.05 indicated consistency). A comparison-adjusted funnel plot was used to determine any publication bias and small-study effects.
Sensitivity analyses, concentrating on the aforementioned effects of laser treatments, were conducted to ascertain whether the results were impacted by the variability in the studies’ characteristics. To examine the robustness of the results, several sensitivity analyses were carried out. These were based on: (i) the subgroup of the initial time-points after surgery to create highly effective prevention of scars; and (ii) the anatomical locations. To demonstrate the statistical significance, 2-sided statistical testing with a p-value of 0.05 was utilized.
RESULTS
A total of 1,967 articles were identified from PubMed, Scopus, Embase, ClinicalTrials.gov, and the Cochrane Library. Of these, 138 duplicated articles were removed. The full texts of 63 publications were assessed, and 1,904 studies were excluded for the reasons shown in Fig. 1. Ultimately, 18 eligible articles were obtained. Thirteen RCTs focused on the effects of the interventions to prevent surgical scars and reported VSS measurements (3, 5, 12–22). The other 7 RCTs were conducted on effects of the interventions to prevent surgical scars and depicted the OSAS measurements (3, 17, 23–27). Two RCTs reported results in both VSS and OSAS score (3, 17) (Table I). The study selection process flow is summarized in a PRISMA flow diagram (Fig. 1). The eligible trials were published between 2003 and 2023. A total of 482 randomized patients with 671 scars were included in the studies, of which 11 trials performed the split-scar technique. Nine trials were conducted in Asia, 3 trials recruited participants from Europe, 2 trials recruited participants from South America, and 4 trials were from North America. Nine studies obtained patients’ age ≥ 20 years. The mean last follow-up time reported for participants was 5.5 months. Thirteen studies excluded patients who had a history of hypertrophic or keloid scars.
| Source | Study design | Location | Age, mean (SD), years | Fitzpatrick skin type | Anatomical site | Assessment | Intervention | Number of patients | Number of scars | Scar age at start | Duration of follow-up |
| Chi et al, (14) 2023 | Observer-blinded, randomized controlled trial | China | NR | NR | Face | VSS | FACL | 10 | 10 | 1 month | 4 months |
| FACL | 10 | 10 | 3 months | 6 months | |||||||
| FACL | 10 | 10 | 6 months | 9 months | |||||||
| Conventional conservative treatment (NS) | 12 | 12 | NR | NR | |||||||
| Kang et al, (25) 2022 | Observer-blinded, randomized controlled trial | USA | 62.5 (13.2) | I–V | Head, neck | POSAS and OSAS | 595nm PDL + 1550nm fractional Er:glass laser | 28 | 28 | 2–8 weeks | 5 months |
| Untreated control | 24 | 24 | |||||||||
| Kim et al, (18) 2022 | Double-blinded, randomized controlled trial | Korea | 42 (7.08) | IV | Neck | VSS | Home-based 830nm LED | 21 | 21 | Not more than 1 week | 6 months |
| 46.9 (10.74) | III | Sham device | 22 | 22 | |||||||
| Cheon et al, (24) 2022 | Observer-blinded, randomized controlled trial | Korea | 48.0 (15.3) | NR | Neck | POSAS and OSAS | 1550nm fractional Er:glass laser + ILSI + Silicone gel (daytime) + Silicone sheet (nighttime) | 32 | 32 | 3 weeks | 6 months |
| 50.3 (13.3) | Silicone gel (daytime) + Silicone sheet (nighttime) | 32 | 32 | ||||||||
| Kim et al, (17) 2021 | Observer-blinded, split-scar, randomized controlled trial | Korea | 62.13 (11.00) | III–IV | Abdomen | OSAS and VSS | 555–950nm IPL + 2940nm fractional Er:YAG laser | 17 | 17 | 1 week | 5 months |
| 2940nm fractional Er:YAG laser | 17 | ||||||||||
| Untreated control | 17 | ||||||||||
| Su et al, (22) 2021 | Observer-blinded, split-scar, randomized controlled trial | China | 30.2 (6.74) | III–V | Various sites | VSS | 500–600nm IPL | 9 | 9 | 2 weeks | 4.5 months |
| Untreated control | 9 | ||||||||||
| Shin et al, (20) 2021 | Observer-blinded, split-scar, randomized controlled trial | Korea | 43.4 (7.4) | NR | Breast | VSS and VAS | FACL | 15 | 15 | 2–3 weeks | 6 months |
| Untreated control | 15 | ||||||||||
| Safra et al, (27) 2019 | Observer-blinded, split-scar, randomized controlled trial | Israel | 51.3 (11.3) | II–IV | Breast | POSAS and OSAS | 595nm PDL + FACL+ Moisturizer + Silicone gel | 18 | 18 | 2–6 weeks | 9 months |
| Moisturizer + Silicone gel | 18 | ||||||||||
| Pongcharoen et al, (19) 2019 | Observer-blinded, split-scar, randomized controlled trial | Thailand | 66.3 (8.92) | III–V | Knee | VSS | 595nm PDL | 39 | 40 | 2 weeks | 6 months |
| Untreated control | 40 | ||||||||||
| Karmisholt et al, (3) 2018_1 | Observer-blinded, split-scar, randomized controlled trial | Denmark | 65.5 (7.88) | I–III | Various sites | OSAS and VSS | 1540nm fractional Er:glass laser | 30 | 30 | Immediately before excision | 4.5 months |
| Untreated control | 30 | ||||||||||
| Karmisholt et al, (26) 2018_2 | Observer-blinded, randomized controlled, intraindividual trial | Denmark | 25.5 (1.56) | II–III | Buttock | OSAS and VAS | 1540nm fractional Er:glass laser | 16 | 144 | 1 day before excision | 3 months |
| Untreated control | 16 | ||||||||||
| Alberti et al, (12) 2017 | Double-blinded, randomized controlled trial | Brazil | 38.2 (9.91) | I–IV | Breast, abdomen | VSS | FACL + Silicone gel | 20 | 20 | 3 weeks | 6 months |
| Silicone gel | 21 | 21 | |||||||||
| Buelens et al, (23) 2017 | Observer-blinded, split-scar, randomized controlled trial | Belgium | 45.46 (10.2) | I–IV | Head, neck | PhGA/PGA and OSAS | FACL | 9 | 9 | Within 3 months after surgery | 6 months |
| Untreated control | 9 | ||||||||||
| Sobanko et al, (21) 2015 | Observer-blinded, split-scar, randomized controlled trial | USA | 63.5 (9.25) | I–IV | Face | VSS and CVAS | FACL | 20 | 20 | 6–7 days | 3 months |
| Untreated control | 20 | ||||||||||
| Kim et al, (16) 2014 | Observer-blinded, split-scar, randomized controlled trial | Korea | 61 (17.04) | III–V | Face, flank | VSS | FACL | 14 | 14 | 2 weeks | 3 months |
| 595nm PDL | 14 | ||||||||||
| Carvalho et al, (13) 2010 | Observer-blinded, randomized controlled trial | Brazil | 47.07 (13.02) | NR | Groin | VSS and VAS and scar thickness measurement | LLLT (830nm diode laser) | 28 | 14 | 1 day | 6 months |
| 47.14 (16.57) | Untreated control | 14 | |||||||||
| Conolouge et al, (15) 2006 | Observer-blinded, split-scar, randomized controlled trial | USA | 59 (13.45) | I–IV | Various sites | VSS | 595nm PDL | 13 | 13 | Immediately after suture removal | 3–5 months |
| Untreated control | 13 | ||||||||||
| Nouri et al., (5) 2003 | Observer-blinded, split-scar, randomized controlled trial | USA | 55 (11.17) | I–IV | Various sites | VSS | 585nm PDL | 11 | 12 | Immediately after suture removal | 3–6 months |
| Untreated control | 12 | ||||||||||
| SD: standard deviation; y: year; NR: not reported; VSS: Vancouver Scar Scale; NS: not specified; FACL: fractional ablative CO2 laser; POSAS: Patient component of the Patient and Observer Scar Assessment Scale; OSAS: Observer component of the Patient and Observer Scar Assessment Scale; PDL: pulsed dye laser; +: combined with; fractional Er:glass laser: fractional erbium:glass laser; LED: light-emitting diode; ILSI: intralesional steroid injection; IPL: intense pulse light; fractional Er:YAG laser: fractional erbium:YAG laser; VAS: visual analogue scale; LLLT: low-level laser therapy; PhGA/PGA: Physician/Patient Global Assessment; CVAS: cosmetic visual analogue scale. | |||||||||||
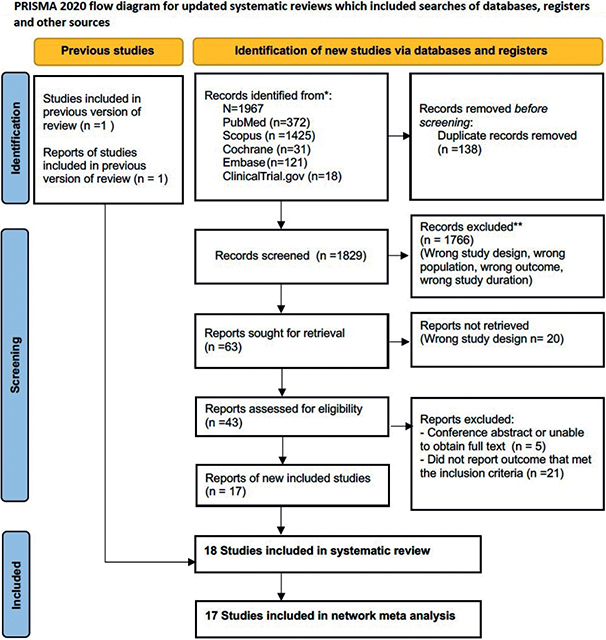
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram summary of the study selection process.
Risk of bias
Appendix S1 provides an overall risk of bias of the RCTs included in the current study. Four studies reported a low risk of bias. Eleven studies had some concerns for risk of bias. In addition, 3 studies showed a high risk of bias, with 2 of them having a high risk of bias for missing outcome data. Complete study characteristics and extracted outcomes data are found at Appendix S1; Figs. S1 and S2.
For network meta-analysis in Vancouver Scar Scale
Thirteen studies, which reported VSS as the primary outcome, were included to the NMA. Of these, the fractional CO2 laser was the most frequently used comparator, studied in 5 out of 13 trials (5/13, 38.4%). There were a total of 9 treatment arms, including 7 lasers and EBDs. Low level laser therapy (LLLT), pulsed dye laser (PDL), fractional erbium:YAG (Er:YAG) laser, intense pulsed light (IPL), IPL+fractional erbium:YAG laser (IPL+ fractional Er:YAG laser), fractional ablative CO2 laser (FACL), fractional erbium glass (Er:glass) laser, silicone gel, and untreated control were studied. Three papers compared FACL with untreated control and PDL to untreated control. LLLT to untreated control was investigated in 2 publications. Fractional Er:glass laser, fractional Er:YAG laser, IPL+ fractional Er:YAG and IPL were compared with untreated control in 1 comparison. FACL was compared with silicone in 1 comparison. In addition, there were comparisons between EBD and laser as IPL+ fractional Er:YAG laser with fractional Er:YAG laser, laser to laser as PDL to FACL and FACL to silicone-based preparation (Fig. 2). The network meta-analysis result of OSAS is illustrated in Appendix S1; Table SV and Fig. S3.
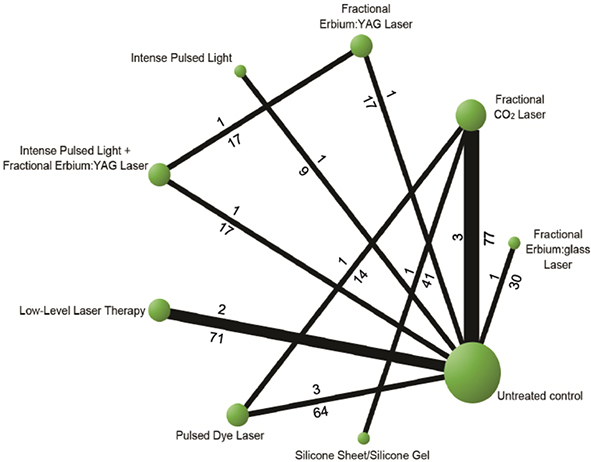
Fig. 2. Networks of all options comparisons for reducton in Vancouver Scar Scale (VSS) score.
Fig. 3 shows the results based on NMA combining direct and indirect comparisons. The treatment associated with the highest reduction in VSS (lower VSS scores imply better scar appearance) was LLLT and PDL –3.78 (95% CI –6.32, –1.24) and –2.46 (95% CI –4.53, –0.38), respectively. Following LLLT and PDL, based on the SURCA value, the results depicted silicone gel –1.75 (95% CI –3.63, 0.14), fractional Er:YAG laser –0.83 (95% CI –2.43, 0.77), FACL –0.66 (95% CI –2.81, 1.49), IPL+ fractional Er:YAG laser –0.27 (95% CI –3.03, 2.49), fractional Er:glass –0.16 (95% CI –1.87, 1.56) and IPL 0.72 (95% CI –2.00, 3.44). Only LLLT and PDL were correlated with a significantly lower weighted mean difference of VSS score for surgical scar prevention compared with other interventions (Appendix S1; Table SIV).
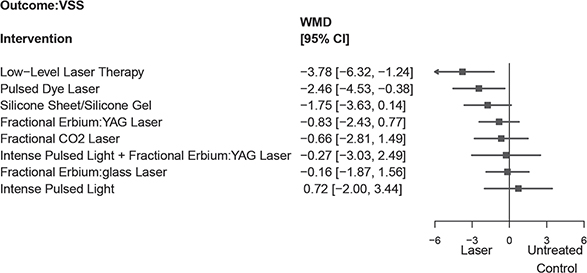
Fig. 3. The summarized results of using laser and energy-based device (EBD) treatment for the reduction in Vancouver Scar Scale (VSS) score. Silicone sheet/gel are also evaluated. WMD: weighted mean difference.
However, there was no significant statistical difference between LLLT and PDL, with –1.32 (95% CI –3.53, 0.89). Six RCTs were used to conduct NMA in OSAS, (3, 17, 23, 24, 26, 27) and the result is shown in Appendix S1; Table SV, Fig. S3. The SURCA rank-bar chart, which illustrates SURCA cumulative probabilities of all outcomes associated with the prevention of surgical scars, is shown in Fig. 4.
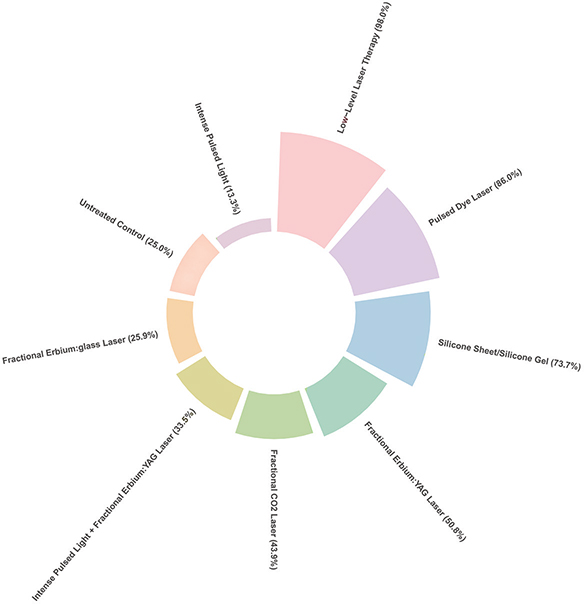
Fig. 4. Rank-bar chart with surface under the cumulative ranking (SUCRA) values for outcomes associated with lasers and energy-based devices used in patients with postsurgical scars.
Optimal period for treatment initiation
Meta-analysis was conducted between the laser/EBD group and non-laser group (consisting of silicone and untreated controls) in both VSS and OSAS (Appendix S1; Figs. S6–S16). Initial treatment times between the laser/EBD groups and the non-laser group were compared in meta-analysis. The forest plot depicted a greater overall decrease in VSS in the group treated within 1 week after surgery than in the group initiating treatment later than 1 week after surgery, with weighted mean difference of –1.51 (–2.43, –0.59) (I2 = 84%) and –0.76 (–1.45, –0.06) (I2 = 76%), respectively (Appendix S1; Fig. S10). Subgroup analysis in trials that performed the interventions within 1 week showed that LLLT had the highest VSS reduction compared with others and untreated control. (Appendix S1; Fig. S17 and Table SVI). Other subgroup and sensitivity analyses were shown in Appendix S1; Figs. S18–S23, Table SVII–SXII.
Adverse effects
Regarding adverse effects, out of the 17 trials included in the study, only minor adverse effects were reported following the interventions. In the PDL treatment group, only minor purpuras were reported and all of them resolved spontaneously (5, 16, 19). No adverse event or significant pain based on the visual analogue score were reported in LLLT group (13, 18). In ablative laser interventions, crusting occurred in 2 patients and resolved in 1 week (17, 20).
Network consistency and small study effects
There was no evidence of any inconsistency in the results of the network meta-analysis in VSS. The comparison-adjusted funnel plots revealed no evidence of small study effects for the VSS score. However, there is an inconsistency in OSAS score as reported in Appendix S1; Table SXIII.
DISCUSSION
Cutaneous scarring is often a major concern for patients following primary closure of surgical wounds. The results of the current study expand on existing systematic reviews (28, 29) and meta-analysis (4), by comparing 8 different laser and EBD treatment interventions and untreated control in a single framework to investigate the benefits of treatment for reducing surgical scar formation. The results confirmed that laser and EBD interventions have the potential to improve the appearance of surgical scars, consistent with previous studies (4, 26). NMA suggests 830nm LLLT and 585nm and 595nm PDL are the most effective interventions among all analysed trials in reducing VSS compared with untreated control and provide highest reduction of VSS of the scars at the last follow-up visits. Although the weighted mean difference between the 2 interventions was not significant, the efficacy of LLLT and PDL was determined to be equally effective. The advantages of LLLT and PDL are related to their non-ablative wounding characteristics and low degree of treatment discomfort, making patients comfortable to start the treatment on the early stage of the scar.
While LLLT and PDL exhibited similar efficacy in reducing scar formation following primary closure of surgical wounds, it is noteworthy that these 2 light sources operate via distinctive mechanisms. LLLT is postulated to modulate the inflammatory and proliferative phases of wound healing through a process known as photobiomodulation (18). In contrast, PDL relies on selective photothermolysis to target the scar’s microvasculature, inducing localized hypoxaemia and consequently influencing collagen production (25). Our understanding suggests that the mechanisms of action employed by LLLT and PDL in the treatment of mature scars are fundamentally rooted in the principles applicable to early scar interventions. Commencing scar treatment during its early stages may potentially mitigate scar formation, particularly when the pathophysiology is immature and amenable to intervention, leading to more substantial enhancements in scar appearance compared with interventions initiated at a later stage.
Although evidence from previous systematic reviews support early intervention as a key to control hyperplastic response leading to hypertrophic scars and keloids, the optimal time to initiate treatment remains controversial. A previous systematic review by Karmisholt et al. (29) reported a wide range of timing for treatment initiation, including the initiation in the inflammatory, proliferative and remodelling phases being effective for significant scar improvement. In contrast, a systematic review and meta-analysis by Kent at al.(4) indicates that laser treatment for minimizing primarily closed surgical scar should be initiated no later than 1 month after operation. However, the most recent systematic review by Behrouz-Pirnia and colleagues (28) could not conclude whether early laser treatment can reduce scar formation, due to the considerable heterogeneity (I2 statistic = 86%) of the currently available evidence. The current analysis further bolsters the trend that LLLT and PDL treatments initiated within 1 week after surgery are associated with a greater decrease in an overall effect on VSS.
Although the current systematic review and NMA focused primarily on closed full-thickness surgical scars for homogeneity, the assumptions can be extrapolated to the other types of scars, including acne, traumatic, and burn scars. The current study only included interventions initiated within 6 months after surgery because it aimed to assess the outcome of early initiation of treatment within the time period that the scar tissue has not yet reached the maturation phase (1). The current study only assessed studies with a follow-up period of at least 3 months postoperatively, in order to ensure that the interventions provided a certain duration of scar mitigation effect.
Silicone-based preparations in the form of sheets and gels are universally considered prophylactic options for hypertrophic scars and keloids. Based on the SURCA value, this NMA showed that silicone-based treatment improves scar appearance to –1.75 (–3.63, 0.14) compared with the untreated control. The mode of action of silicone-based products on scar tissue is unknown, but many agree that it acts at the stratum corneum by reducing evaporation and restores homeostasis, thereby reducing mast cell activity, oedema, vasodilation, and excessive extracellular matrix formation. The authors did not include silicone-based and other topical preparations used as a monotherapy for scar treatment in the current analysis because this systematic review and NMA focused on the use of lasers and EBDs for minimization of surgical scars. Although many RCTs have demonstrated promising outcome of silicone-based preparations in prevention of hypertrophic scar development (30, 31), the effectiveness of silicone in preventing scar formation remains controversial. A systematic review by O’Brien & Jones (32) showed that silicone gel sheeting reduces the incidence of hypertrophic scarring in people prone to scarring compared with untreated control (risk ratio (RR) 0.46, 95% CI 0.21–0.98). However, the studies analysed in this review had a high susceptibility to bias. In contrast, a recent systemic review and meta-analysis evaluating 6 RCTs with a total of 375 patients demonstrated that topical silicone gel significantly reduced pigmentation, height, and pliability scores postoperatively compared with placebos or no treatment (33).
The majority of the RCTs analysed in this systematic review and NMA were monotherapy (using a single laser or EBD). However, in current practice, state-of-the-art treatment for scars is to apply multimodal treatments to maximize the therapeutic outcome. Although not investigated in large RCTs, evidence suggests that combining lasers targeting distinct chromophores (i.e. vascular lasers and non-ablative/ablative fractional lasers) (34), with intralesional and/or topically applied (laser-assisted drug delivery) triamcinolone acetonide (35, 36) and/or 5-fluorouracil (37), can yield superior results.
Study limitations
This NMA has several limitations. First, the heterogeneity of studies may limit the strength of the results. For instance, some studies adjusted for different sets of covariates, and not all studies provided fully adjusted effect estimates; hence the raw data for the meta-analysis was pooled. Secondly, treatment comparison arms were based on a single trial or trials with small sample sizes. Thus, the evidence certainty of the current analysis is limited by the inherent limitations of individual included trials. Thirdly, despite a comprehensive search approach, potential small-study effects exist and might contribute to some of the network effect estimates we observed. Fourthly, several of the studies included in this analysis assessed scar improvement without inclusion of untreated control groups. It is important to acknowledge the possibility that scars may improve over time even in the absence of treatment. Finally, we acknowledge the limited interconnections in the results of the network estimates.
Conclusion
Overall, the results of this systematic review and NMA suggest that low-level laser and pulsed dye laser are similarly effective treatments for minimizing surgical scar formation. There is a trend toward a better therapeutic outcome when treatment is initiated as early as 1 week postoperatively.
REFERENCES
- Profyris C, Tziotzios C, Do Vale I. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part I. The molecular basis of scar formation. J Am Acad Dermatol 2012; 66: 1–10; quiz 11–12.
- Kunishige JH, Katz TM, Goldberg LH, Friedman PM. Fractional photothermolysis for the treatment of surgical scars. Dermatol Surg 2010; 36: 538–541.
- Karmisholt KE, Banzhaf CA, Glud M, Yeung K, Paasch U, Nast A, et al. Laser treatments in early wound healing improve scar appearance: a randomized split-wound trial with nonablative fractional laser exposures vs. untreated controls. Br J Dermatol 2018; 179: 1307–1314.
- Kent RA, Shupp J, Fernandez S, Prindeze N, DeKlotz CMC. Effectiveness of early laser treatment in surgical scar minimization: a systematic review and meta-analysis. Dermatol Surg 2020; 46: 402-410.
- Nouri K, Jimenez GP, Harrison-Balestra C, Elgart GW. 585-nm pulsed dye laser in the treatment of surgical scars starting on the suture removal day. Dermatol Surg 2003; 29: 65–73; discussion 73.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 2021; 372: n71.
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019; 10: Ed000142.
- Higgins J, Sterne J, Savović J, Page MJ, Hróbjartsson A, Boutron I. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Database of Systematic Reviews: Cochrane Methods, London; 2016.
- Kelley GA, Kelley KS. Statistical models for meta-analysis: a brief tutorial. World J Methodol 2012; 2: 27–32.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23: 3105–3124.
- Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med 2017; 12: 103–111.
- Alberti LR, Vicari EF, De Souza Jardim Vicari R, Petroianu A. Early use of CO(2) lasers and silicone gel on surgical scars: prospective study. Lasers Surg Med 2017; 49: 570–576.
- Carvalho RL, Alcantara PS, Kamamoto F, Cressoni MD, Casarotto RA. Effects of low-level laser therapy on pain and scar formation after inguinal herniation surgery: a randomized controlled single-blind study. Photomed Laser Surg 2010; 28: 417–422.
- Chi H, Zhao X, Shen L, Liu Y, Cai M. Optimal timing of fractional CO2 laser on cleft lip scars: a single-blind randomized controlled cohort study. Dermatol Surg 2023; 49: 145–148.
- Conologue TD, Norwood C. Treatment of surgical scars with the cryogen-cooled 595 nm pulsed dye laser starting on the day of suture removal. Dermatol Surg 2006; 32: 13–20.
- Kim DH, Ryu HJ, Choi JE, Ahn HH, Kye YC, Seo SH. A comparison of the scar prevention effect between carbon dioxide fractional laser and pulsed dye laser in surgical scars. Dermatol Surg 2014; 40: 973–978.
- Kim JC, Kang SY, Kim HO, Park CW, Kwon O, Chung BY. Efficacy of combined treatment with intense pulsed light and fractional erbium:YAG laser in scar prevention: a randomized split wound trial. Dermatol Ther 2021; 34: e15061.
- Kim YH, Kim HK, Choi JW, Kim YC. Photobiomodulation therapy with an 830-nm light-emitting diode for the prevention of thyroidectomy scars: a randomized, double-blind, sham device-controlled clinical trial. Lasers Med Sci 2022; 37: 3583–3590.
- Pongcharoen P, Pongcharoen B, Disphanurat W. The effectiveness of a 595 nm pulsed-dye-laser in the treatment of surgical scars following a knee arthroplasty. J Cosmet Laser Ther 2019; 21: 352–356.
- Shin HW, Suk S, Chae SW, Yoon KC, Kim J. Early postoperative treatment of mastectomy scars using a fractional carbon dioxide laser: a randomized, controlled, split-scar, blinded study. Arch Plast Surg 2021; 48: 347–352.
- Sobanko JF, Vachiramon V, Rattanaumpawan P, Miller CJ. Early postoperative single treatment ablative fractional lasing of Mohs micrographic surgery facial scars: a split-scar, evaluator-blinded study. Lasers Surg Med 2015; 47: 1–5.
- Su Q, Wang F, Chai Y, Yan Q, Wang F, Dong Z, et al. A prospective study on the treatment of immediate post-operative scar with narrowband intense pulsed light under polarized dermoscopy. J Cosmet Laser Ther 2021; 23: 137–141.
- Buelens S, Van Hove AS, Ongenae K, Lapeere H, Huvenne W, Vermeersch H, et al. Fractional carbon dioxide laser of recent surgical scars in the head and neck region: a split-scar, evaluator-blinded study. Dermatol Surg 2017; 43: S75–S84.
- Cheon JH, Hwang YJ, Yoon ES, Jung KY, Park SH, Chung JH. Effectiveness of a combination therapy using non-ablative fractional laser and intralesional triamcinolone injection for thyroidectomy scar treatment: a prospective, randomized, blinded pilot study. J Cosmet Dermatol 2022; 21: 2793–2800.
- Kang BY, Ibrahim SA, Weil A, Reynolds KA, Johnson T, Wilson S, et al. Treatment of surgical scars with combination pulsed dye and fractional nonablative laser: a randomized controlled trial. Ann Surg 2022; 276: 975–980.
- Karmisholt KE, Wenande E, Thaysen-Petersen D, Philipsen PA, Paasch U, Haedersdal M. Early intervention with non-ablative fractional laser to improve cutaneous scarring – a randomized controlled trial on the impact of intervention time and fluence levels. Lasers Surg Med 2018; 50: 28–36.
- Safra T, Shehadeh W, Koren A, Salameh F, Friedman O, Sprecher E, et al. Early intervention with pulse dye and CO(2) ablative fractional lasers to improve cutaneous scarring post-lumpectomy: a randomized controlled trial on the impact of intervention on final cosmesis. Lasers Med Sci 2019; 34: 1881–1887.
- Behrouz-Pirnia A, Liu H, Peternel S, Dervishi G, Labeit A, Peinemann F. Early laser intervention to reduce scar formation in wound healing by primary intention: a systematic review. J Plast Reconstr Aesthet Surg 2020; 73: 528–536.
- Karmisholt KE, Haerskjold A, Karlsmark T, Waibel J, Paasch U, Haedersdal M. Early laser intervention to reduce scar formation – a systematic review. J Eur Acad Dermatol Venereol 2018; 32: 1099–1110.
- Chittoria RK, Padi TR. A prospective, randomized, placebo controlled, double blind study of silicone gel in prevention of hypertrophic scar at donor site of skin grafting. J Cutan Aesthet Surg 2013; 6: 12–16.
- Kim JS, Hong JP, Choi JW, Seo DK, Lee ES, Lee HS. The efficacy of a silicone sheet in postoperative scar management. Adv Skin Wound Care 2016; 29: 414–420.
- O’Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2013; 2013: CD003826.
- Wang F, Li X, Wang X, Jiang X. Efficacy of topical silicone gel in scar management: a systematic review and meta-analysis of randomised controlled trials. Int Wound J 2020; 17: 765–773.
- Tao J, Champlain A, Weddington C, Moy L, Tung R. Treatment of burn scars in Fitzpatrick phototype III patients with a combination of pulsed dye laser and non-ablative fractional resurfacing 1550 nm erbium:glass/1927 nm thulium laser devices. Scars Burn Heal 2018; 4: 2059513118758510.
- Majid I, Imran S. Fractional carbon dioxide laser resurfacing in combination with potent topical corticosteroids for hypertrophic burn scars in the pediatric age group: an open label study. Dermatol Surg 2018; 44: 1102–1108.
- Waibel JS, Wulkan AJ, Shumaker PR. Treatment of hypertrophic scars using laser and laser assisted corticosteroid delivery. Lasers Surg Med 2013; 45: 135–140.
- Prince GT, Cameron MC, Fathi R, Alkousakis T. intralesional and laser-assisted 5-fluorouracil in dermatologic disease: a systematic review. J Drugs Dermatol 2018; 17: 274–280.