SHORT COMMUNICATION
Frequency of Acne and Acne Scars in Patients with Neurofibromatosis 1
Monji KOGA and Shinichi IMAFUKU
Department of Dermatology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan. E-mail: mob3mob3@yahoo.co.jp
Citation: Acta Derm Venereol 2024; 104: adv18621. DOI: https://doi.org/10.2340/actadv.v104.18621.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Sep 1, 2023; Accepted: Dec 4, 2023; Published: Jan 10, 2024
Competing interests and funding: The authors have no conflicts of interest to declare.
INTRODUCTION
Neurofibromatosis 1 (NF1) or von Recklinghausen’s disease is inherited as an autosomal dominant genetic disorder (1, 2). The responsible gene, NF1, is known as a tumour suppressor gene and it encodes a protein, neurofibromin, that negatively regulates RAS proteins through the RAS-mitogen-activated protein kinase (MAPK) pathway. Loss of function (LOF) mutation of a single allelic NF1 drives the development of various types of tumours including cutaneous neurofibroma. Furthermore, many non-tumoural comorbidities such as skeletal abnormalities, learning disabilities, attention-deficit-hyperactivity disorder, and some vascular anomalies, are also associated with NF1 (3). Meanwhile, patients with NF1 appear to have some less frequent symptoms and signs than control subjects. In daily practice, many NF1 patients rarely have hypertrophic scars (1, 2, 4, 5), despite repeated surgery on the chest or neck where hypertrophic scars often occur. Moreover, as we have reported previously, neutrophilic inflammatory diseases, such as inflammatory acne or furunculosis, psoriasis, or hidradenitis suppurativa, are rarely seen in patients with NF1 (3). These ‘’negative’’ symptoms and signs should also be attributable to LOF of NF1.
To determine whether patients with NF1 have less frequent inflammatory skin disorders, we chose inflammatory acne as a representative disease and compared lesion numbers.
MATERIALS AND METHODS
This retrospective chart and photograph review study compared NF1 cases and controls. Clinical photographs and clinical information including age, sex and any diagnosed complications, were obtained from medical records. All patient photographs were taken for the purpose of observation during routine record-keeping; using them, acne rash numbers were manually counted and statistically analysed. Rashes were classified into comedones, inflammatory acne, and acne scars. The definition of Hayashi et al. (6) was used as a diagnostic reference.
Cases were patients diagnosed as having NF1, who visited the Department of Dermatology, Fukuoka University Hospital between 2007 and 2021. Diagnosis of NF1 was established according to clinical manifestations, using the diagnostic criteria set by the National Institutes of Health in 1988 (7). Controls were non-NF1 subjects who visited our department with chief complaints other than comedones, inflammatory acne, and acne scars. Patients in the control group had transient or localized skin disorders, such as urticaria, temporary rash, fixed eruption, and facial benign skin tumours. Subjects having side-effects of molecular-targeted drugs or using the mTOR inhibitor sirolimus gel for tuberous sclerosis complex were excluded. The study also excluded patients with a history of systemic disease that could develop inflammatory acne-like lesions, including Behcet’s disease, pyoderma gangrenosum, rosacea, naevus comedonicus, and follicular cysts. After enrollment, control subjects were randomly matched for each age group. As acne usually occurs in patients from adolescence to early adulthood, the age range 10–49 years was set in both groups.
Statistical analysis
JMP Statistical Discovery Software from SAS (SAS Institute, Cary, NC, USA) and EZR (Saitama, Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria), were used (8). Associations were determined using χ2 and multivariate logistic regression analyses. p-values < 0.05 were considered significant.
RESULTS
Seventy-eight NF1 patients (33 males, 45 females) eventually met the inclusion criteria and were enrolled (Table I, Fig. S1). The median age was 25 years (interquartile (IQR) 15–34.5). The control group comprised 102 non-NF1 patients (41 males, 61 females). The median age was 24 years (IQR 18– 37). An age group was created for each 10-year range.
| Subject (n=78) | Control (n=102) | ap-value | |
| Clinical diagnosis (n, %) | NF1: 78 (100) | Benign skin tumour 39 (38.2) | NA |
| Temporary rash 24 (23.5) | |||
| Urticaria 18 (17.6) | |||
| Haemangioma 8 (7.9) | |||
| Subcutaneous nodule 7 (6.8) | |||
| Fixed drug eruption 3 (2.9) | |||
| Median age (IQR) | 25 (15–34.5) | 24 (18–37) | 0.755 |
| Sex (M, W) | 33, 45 | 41, 61 | |
| Age of group, years, n (%) | |||
| 10–19 | |||
| M | 13 (16.6) | 13 (12.7) | 0.819 |
| F | 15 (19.2) | 17 (16.6) | |
| 20–29 | |||
| M | 10 (12.8) | 12 (11.7) | 0.605 |
| F | 11 (14.1) | 18 (17.6) | |
| 30–39 | |||
| M | 5 (6.4) | 7 (6.9) | 0.727 |
| F | 12 (15.3) | 14 (13.7) | |
| 40–49 | |||
| M | 5 (6.4) | 9 (8.8) | 0.941 |
| F | 7 (8.9) | 12 (11.7) | |
| Over all | |||
| M | 33 (42.3) | 41 (40.2) | 0.831 |
| F | 45 (57.6) | 61 (59.8) | |
| aStatistics (χ2 test). | |||
| NA: not applicable; IQR: interquartile range; M: men; W: women. | |||
The mean number of comedones in patients with NF1 was 3.62 (95% confidence interval (95% CI), 2.097~5.159), and in controls it was 3.87 (95% CI 2.365–5.379); differences were not significant (p = 0.824, 95% CI –2.411–1.923) (Table II, Table SI). The mean number of inflammatory acne in patients with NF1 was 0.61 (95% CI 0.121–1.109), while in controls it was 1.41 (95% CI 0.626–2.196); differences were not significant (p = 0.114, 95% CI –1.786–0.195), although there was a trend towards a less frequent occurrence of inflammatory acne in the NF1 group. The mean number of acne scars in NF1 patients was 0.54 (95% CI 0.104–0.972) and for controls it was 2.15 (95% CI 1.309–2.984); here, the difference was significant (95% CI –2.633–0.584, p = 0.0002).
| Comedones | Inflammatory acne | Acne scar | ||||
| aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Sex | 0.600 (0.293~1.230) | 0.165 | 1.180 (0.520~2.690) | 0.689 | 2.860 (1.320~6.200) | *0.007 |
| Age | 0.910 (0.876~0.945) | *< 0.0001 | 0.960 (0.924~0.998) | *0.039 | 1.010 (0.976~1.040) | 0.645 |
| NF1 | 0.957 (0.475~1.930 ) | 0.903 | 0.403 (0.165~0.981) | *0.045 | 0.221 (0.089~0.547) | *0.001 |
| aOR: adjusted odds ratio; 95% CI: 95% confidence interval. *Significant differences at p < 0.05. | ||||||
Multivariate logistic regression analyses adjusted according to sex, age, and the existence of NF1 demonstrated that NF1 was independently associated with inflammatory acne (odds ratio (OR) 0.403, 95% CI 0.165~0.981, p = 0.045) and acne scars (OR 0.221; 95% CI 0.089~0.547, p = 0.001).
DISCUSSION
This study demonstrated the frequency of comedones, inflammatory acne, and acne scars in patients with NF1 compared with controls, in order to evaluate the nature of skin inflammation in patients with NF1.
We found that the prevalence of comedones in patients with NF1 was similar to the control group. However, patients with NF1 had significantly less inflammatory acne by multivariate analyses (p = 0.045). Furthermore, patients with NF1 had significantly fewer acne scars when analysed by either univariate or multivariate analyses. These results suggested that LOF of NF1 did not affect the formation of comedones, but suppressed the formation of inflammatory acne and acne scars.
Selumetinib is an oral selective inhibitor of MAPK kinase (MEK) 1 and 2. Previous clinical trial results for plexiform neurofibroma in children with NF1 demonstrate the cutaneous adverse effects of an acneiform rash (9). Furthermore, trametinib, a MEK1/2 inhibitor approved in Japan for advanced malignant melanoma and small cell carcinoma of the lung, has been reported to cause acneiform eruption as a cutaneous adverse event (10, 11). Moreover, topical sirolimus, an mTOR inhibitor approved for angiofibroma of tuberous sclerosis complex in Japan, is known to produce acneiform rashes as a side-effect (12). Since crosstalk between the RAS-MAPK and mTOR pathways is known, blocking the RAS-MAPK and/or Akt-PI3K pathway may induce acneiform eruption.
There has been little research regarding the immunological findings of NF1. Previously, we found that helper T type-2 (Th2) comorbidities, such as bronchial asthma and atopic dermatitis were more common in NF1 patients than in controls (3). In addition, none of the studied cases had developed hidradenitis suppurativa or psoriasis vulgaris (3). This suggests that patients with NF1 may have an immunological deviation toward Th2 immune responses, and less neutrophilic inflammation.
A retrospective study concluded that patients with NF1 have less postoperative scarring (5). Although scar formation mechanisms are not yet completely understood, inflammation is one of the determining factors (5).
Through studying inflammatory acne, we found a new specific clinical feature: patients with NF1 developed fewer scars, possibly due to lower levels of inflammation in the skin that may be associated with the RAS-MAPK signalling pathway (Fig 1).
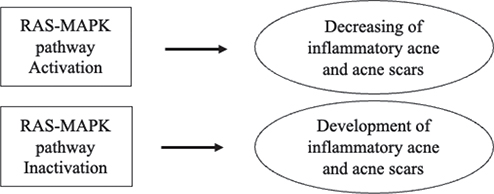
Fig. 1. Hypothetical relationship between the RAS-mitogen-activated protein kinase (MAPK) pathway and inflammatory acne and acne scarring.
Study limitations
Firstly, the sample size was small and all subjects were Japanese; the results may therefore not be generalizable to all races. Secondly, because of its retrospective nature, all evaluations for acne and acne scars were performed using only clinical photographs.
REFERENCES
- Riccardi VM. Neurofibromatosis. Neurol Clin 1987; 5: 337–349.
- Niimura M. Neurofibromatosis in Japan. In: Ishibashi Y, Hori Y, editors. Tuberous sclerosis and neurofibromatosis: epidemiology, pathophysiology, biology and management. Amsterdam: Excerpta Medica; 1990: p. 22–31.
- Koga M, Koga K, Nakayama J, Imafuku S. Anthropometric characteristics and comorbidities in Japanese patients with neurofibromatosis type 1: a single institutional case–control study. J Dermatol 2014; 41: 885–889.
- Yoshida Y, Ehara Y, Koga M, Imafuku S, Yamamoto O. Epidemiological analysis of major complications requiring medical intervention in patients with neurofibromatosis 1. Acta Derm Venereol 2018; 98: 753–756.
- Miyawaki T, Billings B, Har-Shai Y, Agbenorku P, Kokuba E, Moreira-Gonzalez A, et al. Multicenter study of wound healing in neurofibromatosis and neurofibroma. J Craniofac Surg 2007; 18: 1008–1011.
- Hayashi N, Akamatsu H, Kawashima M, Acne Study Group. Establishment of grading criteria for acne severity. J Dermatol 2008; 35: 255–260.
- National Institutes of Health Consensus Development Conference. Neurofibromatosis: Conference statement. Arch Neurol 1988; 45: 575–578.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458.
- Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med 2016; 375: 2550–2560.
- Klesse LJ, Jordan JT, Radtke HB, Rosser T, Schorry E, Ullrich N, et al. The use of MEK inhibitors in neurofibromatosis type 1-associated tumors and management of toxicities. Oncologist 2020; 25: e1109–e1116.
- Anforth R, Liu M, Nguyen B, Uribe P, Kefford R, Clements A, et al. Acneiform eruptions: a common cutaneous toxicity of the MEK inhibitor trametinib. Australas J Dermatol 2014; 55: 250–254.
- Wataya-Kaneda M, Nagai H, Ohno Y, Yokozeki H, Fujita Y, Niizeki H, et al. Safety and efficacy of the sirolimus gel for TSC patients with facial skin lesions in a long-term, open-label, extension, uncontrolled clinical trial. Dermatol Ther (Heidelb) 2020; 10: 635–650.