ORIGINAL REPORT
Effect of Potassium Permanganate on Staphylococcal Isolates Derived from the Skin of Patients with Atopic Dermatitis
Sigrid LUNDGREN1,2 and Andreas SONESSON1,2
1Department of Dermatology and Venereology, Skåne University Hospital and 2Division of Dermatology and Venereology, Department of Clinical Sciences, Lund University, Biomedical Center B14, Lund, Sweden
In atopic dermatitis (AD), Staphylococcus aureus frequently colonizes lesions, leading to superinfections that can then lead to exacerbations. The presence of biofilm-producing isolates has been associated with worsening of the disease. Potassium permanganate is used as a topical treatment of infected eczema, blistering conditions, and wounds. Little is known of its effects against microbes in AD skin. The aim of this study was to explore antibacterial and antibiofilm properties of potassium permanganate against staphylococcal isolates derived from AD skin. Viable count and radial diffusion assays were used to investigate antibacterial effects of potassium permanganate against planktonic staphylococcal isolates. The antibiofilm effects were assessed using biofilm assays and scanning electron microscopy. The Staphylococcus aureus isolates were completely killed when exposed to 0.05% of potassium permanganate. In concentrations of 0.01%, potassium permanganate inhibited bacterial biofilm formation. Eradication of established staphylococcal biofilm was observed in concentrations of 1%. Electron microscopy revealed dense formations of coccoidal structures in growth control and looser formations of deformed bacteria when exposed to potassium permanganate. This suggests antibacterial and antibiofilm effects of potassium permanganate against staphylococcal isolates derived from AD skin, when tested in vitro, and a potential role in the treatment of superinfected AD skin.
Key words: atopic dermatitis; Staphylococcus aureus; Staphylococcus epidermidis; biofilm; bacteria; potassium permanganate; antibacterial.
SIGNIFICANCE
Atopic dermatitis is a common inflammatory skin disease. The bacterium Staphylococcus aureus frequently colonizes the skin, leading to a worsening of the disease. Potassium permanganate is used as a topical treatment of infected eczema, but little is known of its effects. In this study the results showed an antibacterial effect against Staphylococcus derived from the skin of patients with atopic dermatitis. The substance can inhibit biofilm formation but has a weak capacity to eradicate established biofilm when tested in the laboratory. Taken together, potassium permanganate has a potential role in the treatment of superinfected atopic dermatitis skin in reducing the bacterial load.
Citation: Acta Derm Venereol 2024; 104: adv18642. DOI https://doi.org/10.2340/actadv.v104.18642.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
Submitted: Sep 5, 2023; Accepted: Jan 16, 2024; Published: Feb 28, 2024
Corr: Sigrid Lundgren, Department of Dermatology and Venereology, Skåne University Hospital, Lasarettsgatan 15, SE-22185 Lund, Sweden. E-mail: sigrid.lundgren@med.lu.se
Competing interests and funding: AS has received speaker honoraria and consulting fees from AbbVie, LEO Pharma, Pfizer, and Sanofi. Payments were made to AS’s institution. AS has been an investigator for AbbVie.
This work was supported by grants from the Welander and Finsenn Research Foundation (Hudfonden), Skåne University Hospital (SUS) foundations and donations, and the Swedish Government Founds for Clinical Research (ALF).
INTRODUCTION
A topic dermatitis (AD) is one of the most common inflammatory skin diseases; approximately one-fifth of all children gets affected (1) and studies have found a prevalence of adult eczema of 2–10% (2). It is a multifaceted chronic disease caused by a variety of factors including genetic variations, environmental factors, impaired immune response and infections (1). Patients with AD have dysfunctional skin barriers and decreased levels of antimicrobial peptides, making the skin susceptible to bacterial colonization. Studies have shown that up to 90% of these patients are colonized with Staphylococcus aureus in their lesions (3, 4). The severity of the disease is closely connected to the amount of bacterial colonization (5, 6), and the less pathogenic bacteria Staphylococcus epidermidis is found in great abundance in eczematous lesions (7). Although S. epidermidis is considered a beneficial commensal bacterium of the skin microbiota, it is reported that S. epidermidis may trigger inflammation in AD and that biofilm producing isolates could contribute to the aetiology (8, 9).
Biofilms are complex microbially-derived sessile communities where the bacteria are embedded in a matrix of extracellular polymeric substances (10, 11). The formation of biofilm appears to be a survival strategy, within which microorganisms are protected from environmental conditions, antibiotics, antimicrobial peptides and phagocytosis. The endurance in infections caused by biofilm is a huge clinical problem (12). However, most studies concerning skin infections and AD are performed on planktonic bacteria, although recently multiple studies reveal associations between AD and biofilm production by S. aureus (13–15).
Antistaphylococcal substances can play a role in improving AD skin, and antiseptic therapies may be preferable over topical antibiotics due to the risk of antibiotic resistance. Potassium permanganate in concentrations ranging between 0.001% and 0.1% is a widely used substance for external treatment of inflammatory skin diseases and wound management. Although there seem to be gaps in the knowledge of its clinical use and its actual effects against bacteria derived from the skin. The aim of this study was to investigate the antibacterial and antibiofilm effects of potassium permanganate against S. aureus and S. epidermidis isolates derived from AD skin.
MATERIALS AND METHODS
Microorganisms
S. aureus ATCC 29213 and S. epidermidis ATCC 14990 were from the American Type Culture Collection (Rockville, MD, USA). The clinical isolates were derived from lesional skin of patients with AD. The bacterial samples were preferentially taken from the antecubital fossa or from another body location with ongoing eczematous lesion, as described elsewhere (14). The clinical isolates are derived from different patients. The isolates were identified as S. aureus or S. epidermidis by standard routine procedures at the Department of Clinical Microbiology at Skåne University Hospital.
The sampling of skin microbiota from the participants was performed after informed consent, complying with the Declaration of Helsinki, and with approval from the Regional Ethics Examination Board of Lund (permit numbers 144/2010 and 82/2012).
Viable count assay
Viable count assays were performed to evaluate the antibacterial effects of potassium permanganate. S. aureus ATCC 29213 and S. epidermidis ATCC 14990, as well as 3 clinical S. aureus isolates and S. epidermidis isolates from patients with AD were grown to mid-logarithmic phase in 3% tryptic soy broth (TSB). The bacteria were washed and diluted in 10 mM Tris, pH 7.4 to 1×10^7 colony-forming units (cfu)/mL. To mimic the exposure time used in clinical practice the bacteria was then incubated for 10 min together with potassium permanganate in 4 dilutions (0.1%, 0.05%, 0.005% and 0.001%). After potassium permanganate exposure, serial dilutions of the incubation mixture were done in 10 mM Tris, pH 7.4 and then plated on TH-agar plates followed by incubation at 37°C for 24 h before cfu count.
Radial diffusion assay
Radial diffusion assays were performed as previously described (16). An underlay gel composed of 0.03% (w/v) TSB, 1% (w/v) low-electroendosmosis type (low-EEO) agarose (Sigma Aldrich, St Louis, MO, USA) and 0.02% Tween-20 was made. Clinical isolates and reference strains of S. aureus and S. epidermidis [3 x 106 colony-forming units (cfu)] in mid-logarithmic phase were added to this and mixed before being poured into 85 mm Petri dishes. In each gel plate 10 wells (4mm in diameter) were punched out and filled with 5ul of potassium permanganate in 4 different concentrations diluted in dH2O or control (dH2O). Diffusion of the samples occurred during incubation for 3 h at 37°C; thereafter they were covered with 5 mL molten overlay (6% TSB and 1% low-EEO agarose in dH2O). The palates were incubated for 24 h at 37°C before antibacterial effects of potassium permanganate were measured as the formation of clear zones around each well.
Biofilm assay
Examination of the inhibitory effects of potassium permanganate against staphylococcal biofilm formation was performed as described by Hell et al. (17) with some small adjustments. Clinical isolates and reference strains of S. aureus and S. epidermidis were grown in 3% TSB in 37°C while shaking at 180 rpm. When the mid-logarithmic phase was reached, they were washed and diluted in 10 mM Tris, pH 7.4 to 1×10^9 cfu/mL. The aliquots were then further diluted in 1.5% TSB supplemented with 0.3% glucose to 1×10^7 cfu/mL. A 96-well round-bottomed polystyrene microtitre plate (Costar serocluster #2797) was prepared with potassium permanganate in 4 different concentrations (0.01%, 0.05%, 0.1% and 0.5%) and 1.5% TSB supplemented with 0.3% glucose. Bacterial aliquots (5×103 cfu) were added to the wells and the plate was incubated for 22 h in 37°C while shaking at 180 rpm. The wells were washed 3 times with phosphate-buffered saline (PBS), to remove the media and planktonic cells. The biofilms at the bottom of the wells were thereafter stained with 150 μL 0.1% crystal violet solution (Sigma Aldrich) for 15 min at room temperature. The plates were then washed 3 times in PBS before incubation with 99.5% ethanol for 15 min in room temperature to dissolve the dyed biofilm. 100 μL from each well was transferred to a new flat-bottomed 96-well plate where the absorbance, correlating to the amount of biofilm produced, was measured at OD600 in a 96-well plate reader (Victor3 1420 multilabel counter; PerkinElmer, Waltham, MA, USA). The bacterial isolates were tested in 4 independent experiments.
Minimum inhibitory concentration and minimum biofilm eradication concentration
Investigation of the bacterial and biofilm susceptibility to potassium permanganate was performed using a slightly modified version of the Calgary Biofilm Device method (MBECTM Biofilm Inoculator; Innovotech, Edmonton, Canada) (18) following the manufacturer’s protocol. In vitro biofilms were formed when 150 μL bacterial aliquots (106 cfu/mL) in 1.5% TSB supplemented with 0.3% glucose were added to the wells of a MBEC Biofilm Inoculator. Incubation occurred for 24 h in a rotating incubator at 37°C and 180 rpm, which enabled biofilm formation on the lid consisting of pegs. The pegs, now covered with bacterial biofilm, were washed with PBS and shifted to a challenge plate (#167008; Thermo Scientific, Roskilde, Denmark) with serially diluted potassium permanganate in 1.5% TSB supplemented with 0.3% glucose and incubated for 24 h in a rotating incubator at 37°C and 180 rpm. Determination of the minimum inhibitory concentration (MIC), the lowest concentration necessary to inhibit growth of planktonic bacteria, was made by looking for turbidity of the wells indicating bacterial growth. Since potassium permanganate is strongly colouring, 4 drops of 10 μL each from each well were also plated to agar plates and controlled for growth or not after 24 h incubation at 37°C. After exposure to potassium permanganate in the challenge plate, the lid with pegs was washed in PBS and transferred to a recovery plate with wells containing 1.5% TSB supplemented with 0.3% glucose and universal neutralizer according to the manufacturer’s protocol. The plate was sonicated in an ultrasonic bath (Elmasonic S 30H; Elma Hans Schmidbauer GmbH & Co. KG, Singen, Germany) for 10 min at maximum settings enabling the biofilm to disrupt from the pegs down to the wells of the plate. The plate was covered with a flat lid and incubated for 24 h at 37°C. The minimum biofilm eradication concentration, (MBEC), was determined by either reading the turbidity at 600 nm in a 96-well plate reader (Victor3 1420 multilabel counter; PerkinElmer, Waltham, MA, USA) or by bacterial plate counts. Values for MIC and MBEC were recorded as range values from 3 independent experiments. The highest concentration used in this assay was 1% and the serial dilution then gave the following concentrations of 0.5%, 0.25%, 0.12%, 0.06% and 0.03%.
Scanning electron microscopy
To further visualize the effects of potassium permanganate against S. aureus biofilm, a few of the pegs from the Calgary Biofilm Device were examined with scanning electron microscopy (SEM) after exposure to potassium permanganate. For SEM, specimens were fixed with 2.5% glutaraldehyde in 150 mM sodium cacodylate pH 7.2. They were washed with a cacodylate buffer and dehydrated with an ascending ethanol series from 50% (vol/vol) to absolute ethanol. The specimens were then subjected to critical-point drying with carbon dioxide and absolute ethanol was used as an intermediate solvent. The tissue samples were mounted on aluminum holders, sputtered with 20 nm palladium/gold, and examined in a DELPHI PhenomWorld Delmic, Netherlands, correlative light and SEM.
Statistical analysis
Data are presented as means ± standard deviation (SD). To describe differences between groups, Wilcoxon signed-rank test was used, and p<0.05 was considered significant. The statistical software used was GraphPad PRISM8.
RESULTS
Antibacterial effects on planktonic S. aureus and S. epidermidis
In clinical practice, patients are normally subjected to potassium permanganate for 10–20 min. Therefore, this study investigated the in vitro antimicrobial effects against clinical isolates derived from the skin of patients with AD after 10 min exposure to potassium permanganate, in different concentrations ranging from 0.01% to 0.5%. The results showed a partial or total decrease in cfu in all tested staphylococcal isolates. In the lower concentrations of potassium permanganate, the reduction in cfu was less prominent (Fig. 1). This study also determined the MIC values for 10 S. aureus and 1 S. epidermidis isolates derived from patients with AD skin alongside 2 reference strains (Table I).
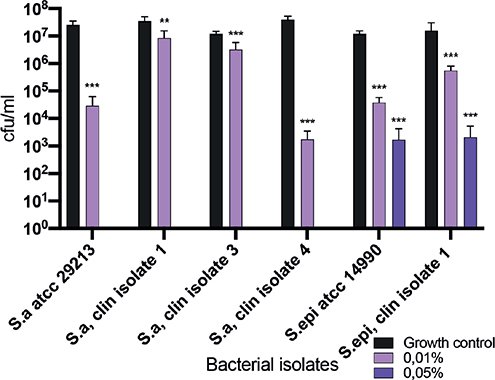
Fig. 1. Antibacterial effects on planktonic S. aureus and S. epidermidis isolates. Clinical S. aureus and S. epidermidis isolates from patients with AD and 2 ATCC strains who have been exposed to potassium permanganate in a concentration range between 0.01% and 0.5% for 10 min, followed by counting of viable colonies. Values indicated with an asterisk are significant (**p ≤ 0.002, ***p ≤ 0.0005) different from growth controls as analysed by Wilcoxon signed-rank test, GraphPad Prism8. Total reduction in colony-forming units (cfu), (no detectable growth) is seen for bacteria exposed to potassium permanganate at concentrations of 0.1% and 0.5%.
Antistaphylococcal activity in radial diffusion assay
Analogous to the disk diffusion tests, which are commonly used to determine antibiotic susceptibility, the antistaphylococcal effects of potassium permanganate on AD-derived isolates were tested in a radial diffusion assay. A dose-dependent inhibition of growth was seen in all clinical S. aureus and S. epidermidis isolates, as well as the reference strains S. aureus ATCC 29213 and S. epidermidis ATCC 14990, when exposed to potassium permanganate. The results indicate that potassium permanganate possesses dose-dependent antibacterial effects against planktonic S. aureus and S. epidermidis when tested in vitro (Fig. 2).
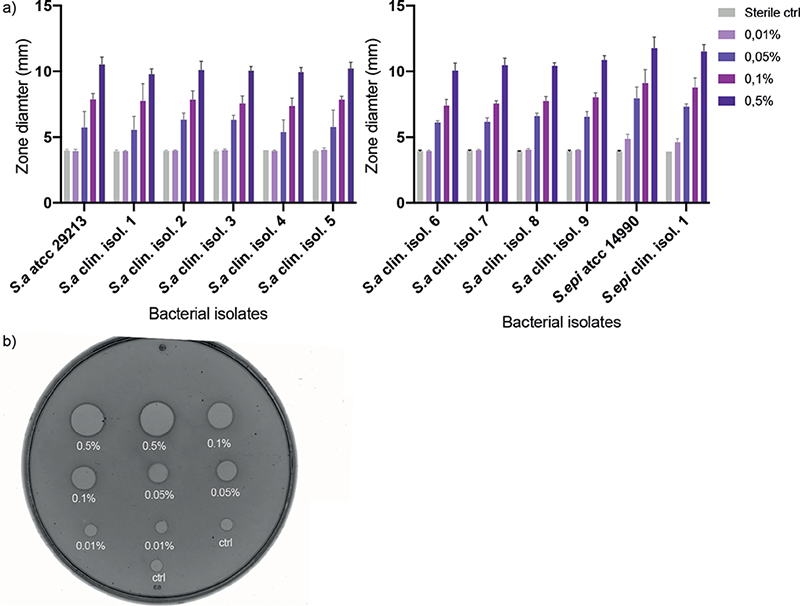
Fig. 2. Inhibition of staphylococcal growth. (a) Antibacterial effects of different concentrations of potassium permanganate against multiple clinical isolates of S. aureus, S. epidermidis, and reference ATCC strains. Results are presented as the mean size of inhibitory zones ± standard deviation (SD), in radial diffusion assay (RDA), of 3 independent experiments. (b) A picture of RDA agar plate with visual clear zones indicating antibacterial effect.
Inhibition of staphylococcal biofilm formation by potassium permanganate
To investigate inhibitory effects against biofilm formation, the clinical isolates and reference ATCC strains were grown in a 96-well microtitre polystyrene plate together with potassium permanganate. Crystal violet was used to stain the biofilm and absorbance was measured that corresponded to the amount of biofilm produced. The results demonstrated that all the tested isolates produced biofilm. The ability to form S. aureus biofilm was reduced in the presence of potassium permanganate and tended to be dose-dependent for most of the bacterial isolates. A majority of the tested isolates showed a decrease in biofilm production when subjected to 0.01% potassium permanganate compared with growth control. The reference ATCC strain of S. epidermidis showed a similar pattern as the S. aureus strains; however, the clinical isolate of S. epidermidis produced a low amount of biofilm and the presence of potassium permanganate exhibited minor anti-biofilm impact (Fig. 3).
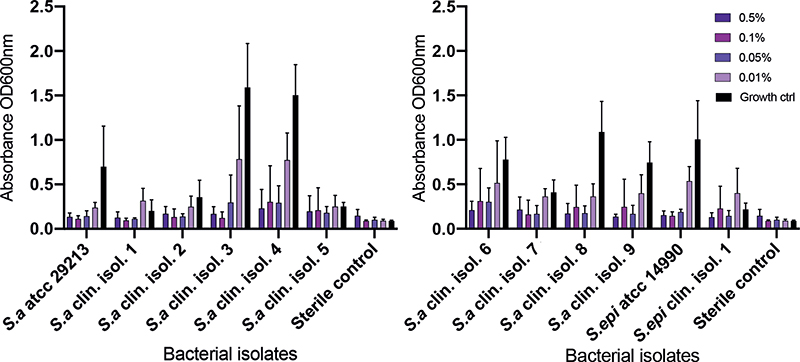
Fig. 3. Inhibition of biofilm formation. Production of S. aureus and S. epidermidis biofilm in the presence of potassium permanganate. After 24 h the biofilms were stained with crystal violet, washed, and dissolved in ethanol; the absorbance was read with enzyme-linked immunoassay (ELISA). Results are shown as mean absorbance (OD600) ± standard deviation (SD), of 3 independent experiments, corresponding to the quantity of biofilm produced. For the vast majority of the isolates a dose-dependent reduction in biofilm formation was seen.
Effect of potassium permanganate against established staphylococcal biofilm
To investigate the capacity of potassium permanganate to eradicate established staphylococcal biofilms, this study used a modified version of the Calgary Biofilm Device method. Biofilms were formed on plastic pegs and then exposed to potassium permanganate in concentrations between 0.03% and 1%. The results showed a weak effect against S. aureus biofilm in the tested concentrations with MBEC values ranging from 1% to above the highest tested concentration of 1%. The corresponding effect against S. epidermis biofilms demonstrated MBEC values of 0.25% to 1% (Table I). Further examinations of the plastic pegs were made with SEM. The results showed densely packed coccoid bacteria on the surface of the pegs in growth controls, with fewer as the concentrations increased. In the reference S. aureus ATCC strain, the highest concentration of 1% exhibited malformed and broken bacterial structures (Fig. 4).
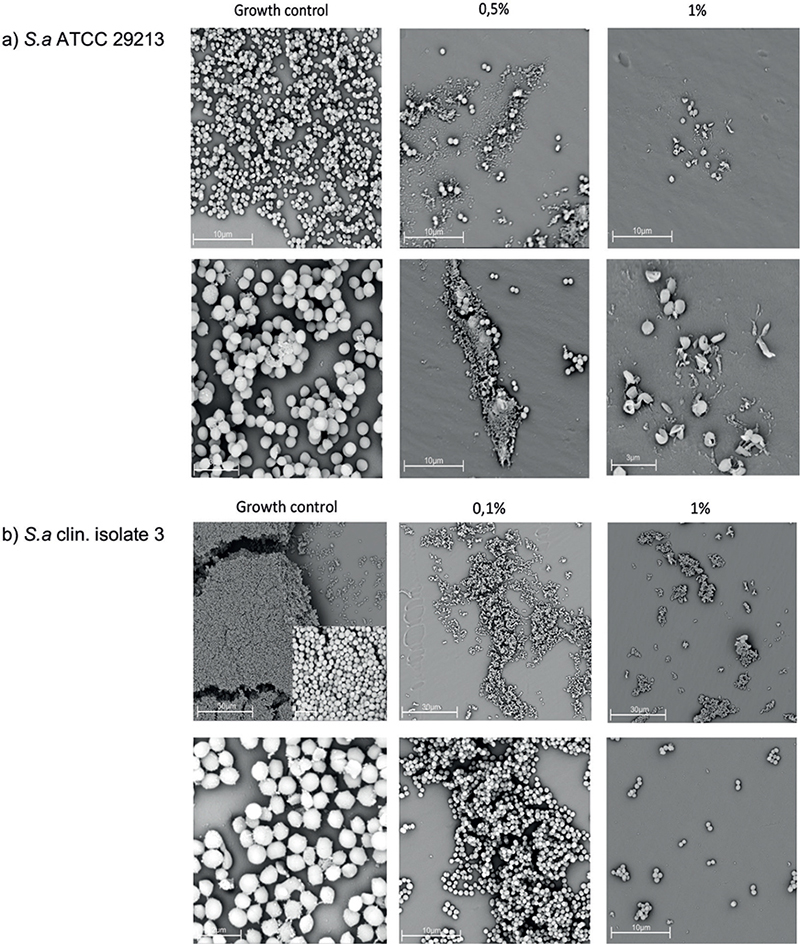
Fig. 4. Scanning electron microscopy (SEM) of potassium permanganate-treated staphylococcal communities. SEM examination of the distribution of bacterial biofilm on plastic pegs after treatment with potassium permanganate in different concentrations. With a higher concentration of potassium permanganate, fewer bacteria are seen. (a) S. aureus ATCC 29213. The rounded structures of the bacteria are altered in 1% concentration. (b) S. aureus clinical isolate 3.
DISCUSSION
Potassium permanganate is a frequently used topical treatment for AD skin in concentrations ranging from 0.001% and 0.1% depending on the severity of the eczematic lesions or if the patient is a child or an adult. Although used for many decades, its effect against bacteria derived from AD skin is scarcely investigated. There is only 1 study from 1992 where Stadler, et al. show a reduction in surviving microbes when bacteria from eczematic patients were exposed to potassium permanganate in fluid (19). In the current study, we can confirm a similar reduction in planktonic S. aureus when treated with potassium permanganate, in vitro, in clinically relevant doses for 10 min, as seen in the viable count assay. There is a known correlation between high S. aureus density on AD skin and increased disease severity score (SCORAD) (20), indicating that a reduction in the bacterial load is of importance for the healing of the lesions and restoring the skin barrier.
Biofilms are known to be a survival strategy for bacteria, to shield them from environmental conditions, often making them difficult to reduce when established (21). The ability of S. aureus to form bacterial biofilm in AD skin has been linked to a worsening of the disease (3). Earlier we demonstrated the presence of bacterial biofilm on the surface of AD skin with SEM of biopsies from AD skin (14, 22). Previous data has shown some antiseptics, such as sodium hypochlorite, to exert an ability to reduce biofilm formation and demolish established staphylococcal biofilm in vitro and ex vivo (22). This concept is thought to play a role in topical treatment of AD skin with S. aureus biofilm present. To our knowledge no study has previously been performed examining whether potassium permanganate possesses antibiofilm effects against staphylococcal strains derived from AD skin.
These data show that concentrations of 0.01% potassium permanganate can inhibit staphylococcal biofilm formation in vitro. These findings are supportive of the anecdotally reported beneficial effects of potassium permanganate as a topical treatment of infected eczema. However, when we investigated the ability to demolish established staphylococcal biofilm in vitro, the effect was weak; in some cases a concentration of 1% could exhibit antibiofilm effects, but often the biofilm endured even this intensity. These findings suggest that potassium permanganate should not be considered as an antibiofilm treatment in AD. In a clinical study on leg ulcers, where the microbial ulcer flora was investigated after treatment with wet gauze dressings soaked in antiseptic solutions, potassium permanganate 0.015% reduced the mean number of bacteria per ulcer in the 10 ulcers investigated, but the results were not significant (23). However, antiseptic solutions, such as potassium permanganate, might speculatively have more favourable effects when applied to AD skin by reducing the bacterial contamination. Irritation and skin damage are reported to occur, especially if potassium permanganate is used in tablet form (24, 25), and it is therefore important to conduct clinical studies to clarify these risks. Despite this, it is conceivable that, as the skin of patients with AD is largely intact in most places, a slightly higher concentration of potassium permanganate with a more potent antibacterial effect might be tolerated without irritative or toxic reactions. However, these questions must be further evaluated in vivo in clinical trials to elucidate the optimal concentration of potassium permanganate to be used clinically.
In summary, these findings showed an antibacterial effect of potassium permanganate, in vitro, against S. aureus and S. epidermidis derived from the skin of patients with AD. The substance has the ability to inhibit biofilm formation, but has a weak capacity to eradicate established biofilm, in vitro. Taken together, potassium permanganate has a potential role in the treatment of AD skin to reduce bacterial load.
REFERENCES
- Weidinger, S. and N. Novak. Atopic dermatitis. Lancet 2016; 387: 1109–1122.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol 2013; 132: 1132–1138.
- Allen HB, Vaze ND, Choi C, Hailu T, Tulbert BH, Cusack C, et al. The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol 2014; 150: 260–265.
- Park HY, Kim CR, Huh IS, Jung MY, Seo EY, Park JH, et al. Staphylococcus aureus colonization in acute and chronic skin lesions of patients with atopic dermatitis. Ann Dermatol 2013; 25: 410–416.
- Breuer K, S HA, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol 2002; 147: 55–61.
- Goh CL, Wong JS, Giam YC. Skin colonization of Staphylococcus aureus in atopic dermatitis patients seen at the National Skin Centre, Singapore. Int J Dermatol 1997; 36: 653–657.
- Bjerre R.D, Bandier J, Skov L, Engstrand L, Johansen JD. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol 2017;177: 1272–1278.
- Ochlich D, Rademacher F, Drerup KA, Glaser R, Harder J. The influence of the commensal skin bacterium Staphylococcus epidermidis on the epidermal barrier and inflammation: Implications for atopic dermatitis. Exp Dermatol 2023; 32: 555–561.
- Allen HB, Mueller JL. A novel finding in atopic dermatitis: film-producing Staphylococcus epidermidis as an etiology. Int J Dermatol 2011; 50: 992–993.
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002; 15: 167–193.
- Wells CL, Henry-Stanley MJ, Barnes AM, Dunny GM, Hess DJ. Relation between antibiotic susceptibility and ultrastructure of Staphylococcus aureus biofilms on surgical suture. Surg Infect (Larchmt) 2011; 12: 297–305.
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284: 1318–1322.
- Di Domenico EG, Cavallo I, Bordignon V, Prignano G, Sperduti I, Gurtner A, et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: a pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci Rep 2018; 8: 9573.
- Sonesson A, Przybyszewska K, Eriksson S, Morgelin M, Kjellstrom S, Davies J, et al. Identification of bacterial biofilm and the Staphylococcus aureus derived protease, staphopain, on the skin surface of patients with atopic dermatitis. Sci Rep 2017; 7: 8689.
- Gonzalez T, Stevens ML, Baatyrbek Kyzy A, Alacron R, He H, Kroner JW, et al. Biofilm propensity of Staphylococcus aureus skin isolates is associated with increased atopic dermatitis severity and barrier dysfunction in the MPAACH pediatric cohort. Allergy 2021; 76: 302–313.
- Sonesson, A, Kasetty G, Olin AL, Malmsten M, Morgelin M, Sorensen OE, et al. Thymic stromal lymphopoietin exerts antimicrobial activities. Exp Dermatol 2011; 20: 1004–1010.
- Hell E, Giske CG, Nelson A, Romling U, Marchini G. Human cathelicidin peptide LL37 inhibits both attachment capability and biofilm formation of Staphylococcus epidermidis. Lett Appl Microbiol 2010; 50: 211–215.
- Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A.The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 1999; 37: 1771–1776.
- Stalder JF, Fleury M, Sourisse M, Allavoine T, Chalamet C, Brosset P, et al. Comparative effects of two topical antiseptics (chlorhexidine vs KMn04) on bacterial skin flora in atopic dermatitis. Acta Derm Venereol 1992; 176: 132–134.
- Guzik TJ, Bzowska M, Kasprowics A, Czerniawska-Mysik G, Wojcik K, Szmyd D, et al. Persistent skin colonization with Staphylococcus aureus in atopic dermatitis: relationship to clinical and immunological parameters. Clin Exp Allergy 2005; 35: 448–455.
- Vlassova N, Han A, Zenilman JM, James G, Lazarus GS. New horizons for cutaneous microbiology: the role of biofilms in dermatological disease. Br J Dermatol 2011; 165: 751–759.
- Eriksson S, vand der Plas MJ, Morgelin M, Sonesson A. Antibacterial and anti-biofilm effects of sodium hypochlorite against Staphylococcus aureus isolates derived from patients with atopic dermatitis. Br J Dermatol 2017; 177: 513–521
- Hansson C, Faergemann J. The effect of antiseptic solutions on microorganisms in venous leg ulcers. Acta Derm Venereol 1995; 75: 31–33.
- Baron S, Moss C. Caustic burn caused by potassium permanganate. Arch Dis Child 2003; 88: 96.
- Gelmetti C. Skin cleansing in children. J Eur Acad Dermatol Venereol 2001; 15: 12–15.