ORIGINAL REPORT
Effects of Psychological Stress on Spontaneous Itch and Mechanical Alloknesis of Atopic Dermatitis
Qiaofeng ZHAO1, Mitsutoshi TOMINAGA1, Sumika TOYAMA1, Eriko KOMIYA1, Tomohiro TOBITA1, Motoki MORITA1, Ying ZUO1, Kotaro HONDA1, Yayoi KAMATA1 and Kenji TAKAMORI1,2
1Juntendo Itch Research Center (JIRC), Institute for Environmental and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, and 2Department of Dermatology, Juntendo University Urayasu Hospital, Chiba, Japan
Atopic dermatitis (AD), a chronic inflammatory skin disease, manifests as an intractable itch. Psychological stress has been suggested to play a role in the onset and worsening of AD symptoms. However, the pathophysiological relationships between psychological stressors and cutaneous manifestations remain unclear. To elucidate the mechanisms underlying the stress-related exacerbation of itch, we investigated the effects of water stress, restraint stress and repeated social defeat stress on itch-related scratching behaviour, mechanical alloknesis and dermatitis in male NC/Nga mice with AD-like symptoms induced by the repeated application of ointment containing Dermatophagoides farina body. NC/Nga mice with AD-like symptoms were subjected to water stress, restraint stress and repeated social defeat stress, and their scratching behaviour, sensitivity to mechanical stimuli (mechanical alloknesis) and severity of dermatitis were evaluated. Social defeat stress+ Dermatophagoides farina body-treated mice exposed to stress showed slower improvements in or the exacerbation of AD-like symptoms, including dermatitis and itch. In the mechanical alloknesis assay, the mechanical alloknesis scores of social defeat stress+ Dermatophagoides farina body-treated mice exposed to stress were significantly higher than those of non-exposed social defeat stress+ Dermatophagoides farina body- and social defeat stress-treated mice. These results suggest that psychological stress delays improvements in dermatitis by exacerbating itch hypersensitivity in AD.
Key words: atopic dermatitis; corticosterone; itch; mechanical alloknesis; psychological stress.
SIGNIFICANCE
Atopic dermatitis, a chronic inflammatory skin disease, manifests as an intractable itch; however, the underlying mechanisms remain unclear. Although psychological stress plays a crucial role in atopic dermatitis, this relationship has not yet been elucidated in detail and there is currently no information on stress elicited by the different effects of atopic dermatitis. The present study successfully created an appropriate model to examine the relationship between stress and the development of atopic dermatitis. This model will provide insights into the pathogenesis of atopic dermatitis and contribute to the development of therapeutic and preventive methods that target stress-induced itch exacerbation and hypersensitivity.
Citation: Acta Derm Venereol 2024; 104: adv18685. DOI https://doi.org/10.2340/actadv.v104.18685.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Sept 11, 2023; Accepted: Feb 15, 2024; Published: Apr 2, 2024
Corr: Kenji Takamori, MD, PhD, Juntendo University Graduate School of Medicine, 2-1-1 Tomioka, Urayasu, Chiba 279-0021, Japan. E-mail: ktakamor@juntendo.ac.jp
Competing interests and funding: The authors have no conflicts of interest to declare.
The present study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI: a Grant-in-Aid for Early-Career Scientists (Grant number 23K15292) and a Grant-in-Aid for Scientific Research (B) (Grant numbers 20H03568 and 22H02956).
INTRODUCTION
A topic dermatitis (AD) is one of the most common, chronic inflammatory-relapsing skin diseases that affects up to 20% of children and 3% of adults. The prevalence and incidence of AD have increased in recent years. The dysregulation of the immune system plays a key role in the pathophysiology of AD, causing inflammation, pruritus and skin barrier dysfunction (1). Chronic itch in AD significantly reduces the quality of life of patients and causes disease burden (2).
Most patients with AD have elevated serum levels of IgE. Additionally, the infiltration of immune cells, including CD4+ T cells, eosinophils and mast cells, has been reported, suggesting Th2 cell-mediated inflammation activated by interleukin (IL)-4 and/or IL-13 (3). Furthermore, IL-4 and IL-13 play an important role in the pathogenesis of AD, orchestrating effector Th2 immune responses that contribute to the development and progression of the disease (4). However, IL-4R antibodies and JAK inhibitors were recently shown to reduce the itchiness of AD and, thus, have become an important component in treatment in clinical practice (5).
Psychological stress has been suggested to play a role in the onset and worsening of AD symptoms in clinical practice. The hypothalamic–pituitary–adrenal (HPA) axis is crucially involved in the adaptive response to stressors (6). Following exposure to stress, the up-regulation of neuropeptide mediators in the brain, endocrine organs and peripheral nervous system directly affects immune cells in the skin, which may contribute to the exacerbation of AD (7). However, the precise mechanisms linking psychological stressors to the development of skin manifestations in AD remain unclear. Therefore, the present study investigated the effects of three different types of stress: water stress (WS), restraint stress (RS) and repeated social defeat stress (R-SDS), on itch-related scratching behaviour, sensitivity to mechanical stimuli (mechanical alloknesis) and the severity of dermatitis in male NC/Nga mice with AD-like symptoms.
MATERIALS AND METHODS
Animals
Male NC/Nga (Oriental Yeast Tokyo, Japan) mice aged 7 to 8 weeks and male ICR mice aged 10 to 12 weeks were obtained from Japan Charles River Laboratory (Yokohama, Japan). All animals were housed in the experimental animal facility of Juntendo University Graduate School of Medicine under a 12 h light:dark cycle at a regulated temperature of 22–24°C, with food and tap water provided ad libitum. All animal procedures were approved by the Animal Care and Use Committee of Juntendo University Graduate School of Medicine. The present study conformed to the National Institute for Health guidelines for animal research.
Induction of atopic dermatitis-like symptoms in NC/Nga mice
Cutaneous barrier disruption was achieved by the application of 150 μl 4% sodium dodecyl sulphate (SDS) to shaved dorsal skin 2 h before the application of ointment containing Dermatophagoides farina body (Dfb) (Biostir AD®, Biostir Inc., Osaka, Japan). This procedure was repeated twice a week for 3 weeks to produce SDS+Dfb-treated mice. Scratching behaviour was measured and quantified using the SCLABA®-Next System (Noveltec, Kobe, Japan) for 12 h as previously reported (8).
Skin lesions were scored as follows: the severity of (1) erythema/haemorrhage, (2) scarring/dryness, (3) oedema and (4) excoriation/erosion was assessed with scores of 0 (none), 1 (mild), 2 (moderate) and 3 (severe). The total skin score was calculated as the sum of individual scores (9).
Stress loading on mice
WS: SDS+Dfb-treated mice were placed into a plastic tank (45 cm length × 25 cm width × 25 cm height) containing warm water (35~36°C, depth of 5 to 6 cm) and removed after 2 h as previously reported (10). The procedure was repeated each weekday for 2 weeks.
RS: SDS+Dfb-treated mice were separately enclosed in a plastic tube (30 mm diameter × 114 mm length, capacity of 50 ml) with several small holes for ventilation as previously reported (11). During the 2 h restraint, mice were deprived of food and water. The procedure was repeated each weekday for 2 weeks.
R-SDS: SDS+Dfb-treated mice were physically exposed to ICR mice (aggressor) for 10 minutes each weekday for 2 weeks. After the exposure period, the two mice were separated by a metal mesh in the same cage for 24 h to expose them to sensory stress without physical contact as previously reported (12).
Assessment of urine corticosterone levels using ELISA
Urine samples were collected after the AD induction phase in the 3rd week and the stress loading phase during the 1st and 2nd weeks. Urine samples were collected 2 h after the last stress loading, and were then stored in 2-ml tubes at −80 °C until analysed. A volume of 1.5 μl was used and diluted at a ratio of 1:20 into a diluent for the analysis. Urine corticosterone levels were measured using the AssayMax™ Corticosterone ELISA kit (Assaypro, MO, USA) according to the manufacturer’s instructions.
Mechanical alloknesis assay
The mechanical alloknesis assay was performed according to a previously reported method (13). Briefly, von Frey filaments were applied to shaved skin and held in place for up to 1 second. One stimulus was delivered with a 5-second interval, and this mechanical itch was repeated 30 times using von Frey filaments with a bending force of 0.07 g or 0.16 g (Fig. S1e).
Statistical analysis
All experiments were repeated at least three times. Data are expressed as mean values ± the standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA), with p < 0.05 indicating a significant difference. Data were analysed by a two-way ANOVA and expressed as the mean ± SEM.
RESULTS
Induction of atopic dermatitis-like symptoms
The SDS+Dfb treatment was applied twice a week to the dehaired back skin of mice for 3 weeks, which induced AD-like symptoms (Fig. S1a). Mice were then subjected to the following psychological stress loading: WS, RS, and R-SDS (Fig. S1b–d).
Effects of psychological stress on urine corticosterone levels and the relative ratio of body weight
After the SDS+Dfb treatment for 3 weeks, stress loading was performed once daily for 2 weeks, and the urinary level of corticosterone, a stress marker, was measured using an ELISA on weeks 1 and 2 (Fig. S1a). In the 1st week, urine corticosterone levels were significantly higher in SDS+Dfb+RS- and SDS+Dfb+R-SDS-treated mice than in SDS+Dfb-treated mice (Fig. 1a). In the 2nd week, urine corticosterone levels were markedly higher in SDS+Dfb+R-SDS-treated mice than in SDS+Dfb-treated and SDS+Dfb+WS-treated mice (Fig. 1a). However, in the 1st and 2nd weeks, no significant differences were observed between SDS+Dfb-treated mice and SDS+Dfb+WS-treated mice (Fig. 1a).
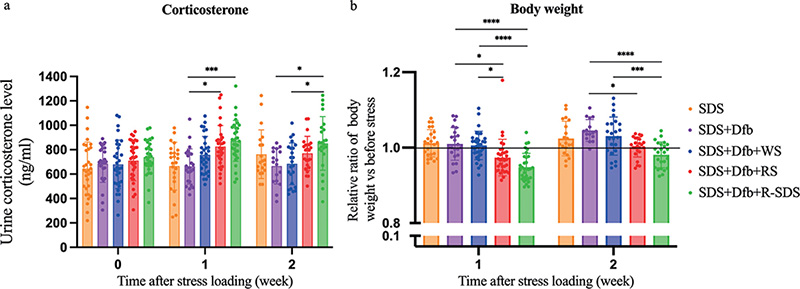
Fig. 1. Effects of psychological stress on urine corticosterone levels and the relative ratio of body weight. (a) In the 1st week, urine corticosterone levels were significantly higher in SDS+Dfb+RS- and SDS+Dfb+R-SDS-treated mice than in SDS+Dfb-treated mice. In the 2nd week, they remained significantly higher in SDS+Dfb+R-SDS-treated mice than in both SDS+Dfb-treated mice and SDS+Dfb+WS-treated mice. (*p < 0.05, ***p < 0.0005, n = 25–32). (b) The relative ratio of body weight was lower in SDS+Dfb-treated mice exposed to a stressor than in those not exposed to any of the three stressors. In the 1st and 2nd weeks, it was significantly lower in SDS+Dfb+RS- and SDS+Dfb+R-SDS-treated mice than in SDS+Dfb-treated mice. In the 1st and 2nd weeks, the relative ratio of body weight was significantly lower in SDS+Dfb+R-SDS-treated mice than in SDS+Dfb+WS-treated mice. Additionally, a comparison of SDS+Dfb+RS-treated and SDS+Dfb+WS-treated mice in the 1st week showed a significant decrease (*p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001, n = 23–32). Numbers represent the mean ± SEM of eight independent experiments. (The statistical analysis did not include the SDS-treated mice.)
The relative body weight ratio was obtained by dividing body weight after stress in the 1st or 2nd week by body weight before stress (Fig. S1a). The relative ratio of body weight was lower in SDS+Dfb-treated mice exposed to a stressor than in those not exposed to any of the three stressors (Fig. 1b). In the 1st and 2nd weeks, the relative ratio of body weight was significantly lower in SDS+Dfb+RS-treated and SDS+Dfb+R-SDS-treated mice than in SDS+Dfb-treated mice (Fig. 1b). Furthermore, in the 1st and 2nd weeks, it was significantly lower in SDS+Dfb+R-SDS-treated mice than in SDS+Dfb+WS-treated mice (Fig. 1b). Additionally, a significant decrease was evident in comparisons of SDS+Dfb+RS- and SDS+Dfb+WS-treated mice in the first week (Fig. 1b).
Effects of psychological stress on skin inflammation and spontaneous itch in AD-NC/Nga mice
SDS+Dfb NC/Nga mice exposed to psychological stress showed a slow improvement and R-SDS psychological stress worsened AD-like symptoms in NC/Nga mice (Fig. S2a).
The relative dermatitis score/scratching bout ratio was obtained by dividing dermatitis scores/scratching bouts after stress in the 1st or 2nd week by dermatitis scores/scratching bouts (Fig. S1a). In the 1st and 2nd weeks of the present study, SDS+Dfb-treated mice exposed to stress exhibited a higher relative ratio of dermatitis scores than those not exposed to stress. The relative ratio of dermatitis scores in SDS+Dfb+R-SDS-treated mice differed significantly from that in SDS+Dfb-treated mice, and this effect was observed in both the 1st and 2nd weeks (Fig. S2b). Additionally, during the 1st week, SDS+Dfb-treated mice exposed to R-SDS displayed a significantly higher relative ratio of scratching bouts than SDS+Dfb- and SDS+Dfb+WS-treated mice, suggesting an enhancement in the impact of AD under psychological stress conditions (Fig. S2c).
Effects of psychological stress on mechanical alloknesis
During the induction phase of AD, mechanical alloknesis scores were significantly higher in SDS+Dfb-treated mice at 0.07 g and 0.16 g than in naive and SDS-treated mice (Fig. 2a, b). Moreover, in the stress loading phase, SDS+Dfb-treated mice exposed to stress had significantly higher mechanical alloknesis scores at 0.07 g and 0.16 g than in those in mice not exposed to any stress (Fig. 2c, d).
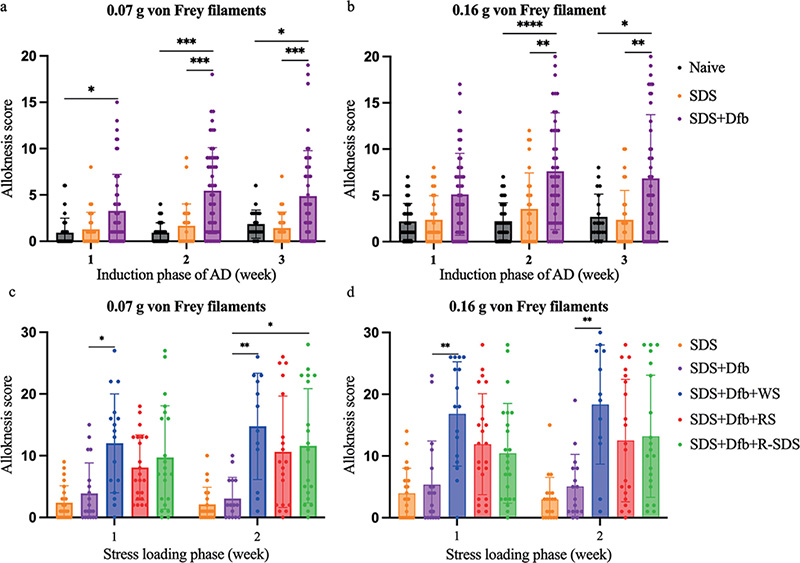
Fig. 2. Effects of psychological stress on mechanical alloknesis. Alloknesis scores were significantly higher in SDS+Dfb-treated mice at 0.07 g (a) and 0.16 g (b) than in naive and SDS-treated mice (in the induction phase of AD, *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.0001, n = 28–52). SDS+Dfb-treated mice exposed to stress had significantly higher alloknesis scores at 0.07 g (c) and 0.16 g (d) (the statistical analysis did not include SDS-treated mice) than mice not exposed to any stress (in the stress loading phase, *p < 0.05, **p < 0.005, n = 16–23). Numbers represent the mean ± SEM of 6 independent experiments.
Relationship between urine corticosterone levels and mechanical alloknesis scores under different stress-induced conditions
In the stress loading phase, a thorough analysis revealed a correlation between urine corticosterone levels and mechanical alloknesis scores in SDS+Dfb+WS-treated mice at 0.07 g (Fig. 3a) and in SDS+Dfb+R-SDS-treated mice at 0.07 g and 0.16 g (Fig. 3c). However, a weak correlation was observed in SDS+Dfb+WS-treated mice at 0.16 g (Fig. 3a) or in SDS+Dfb+RS-treated mice at 0.07 g and 0.16 g (Fig. 3b).
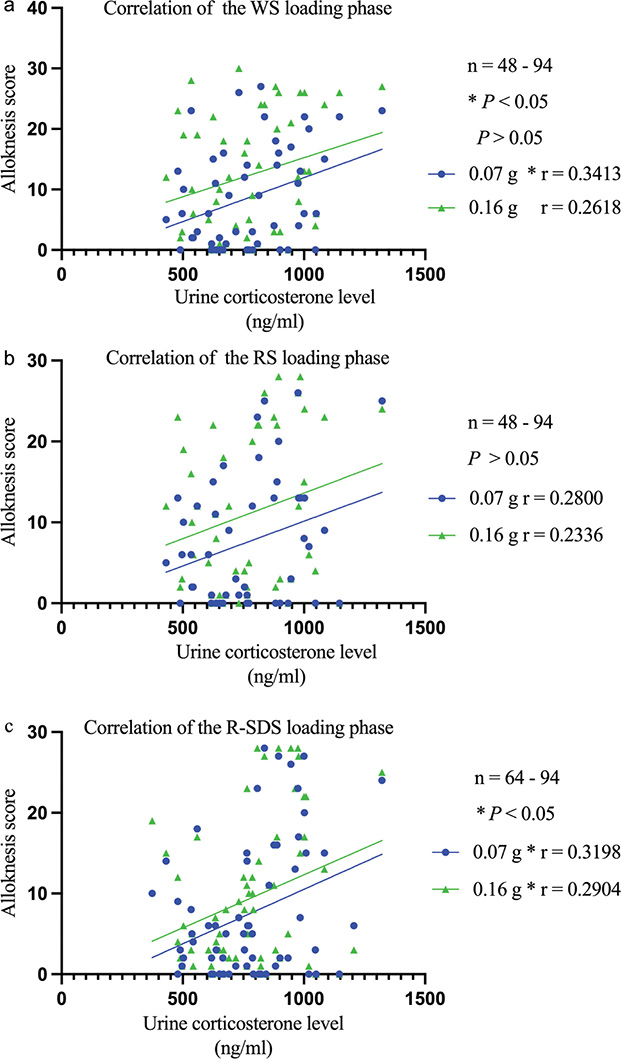
Fig. 3. Urine corticosterone levels correlate with mechanical alloknesis scores in mice with AD-like symptoms. In the stress loading phase, a correlation was observed between urine corticosterone levels and mechanical alloknesis scores in SDS+Dfb+WS-treated mice (0.07 g) and SDS+Dfb+R-SDS-treated mice (0.07 g and 0.16 g, *p<0.05 a and c). However, a weak correlation was found in SDS+Dfb+WS-treated mice (0.16 g) or SDS+Dfb+RS-treated (0.07 g and 0.16 g, p > 0.05 a and b).
DISCUSSION
In the present study, we found that SDS+Dfb-treated mice exposed to psychological stress, particularly R-SDS, showed slower improvements in AD-like symptoms, including dermatitis and itch-related scratching behaviour (Fig. S2b and c). The HPA axis has been identified as the major pathway through which the central nervous system exerts control over the immune system under stressful conditions, affecting immune responses, cytokine production, mast cell activity, neuropeptide signalling and the skin barrier (14). The delay observed in symptom improvement is consistent with previous findings on the formation of itch–scratch cycles, showing that itching exacerbated dermatitis (7).
Psychological stress has been suggested to play a significant role in the pathogenesis and clinical course of AD skin conditions (15). However, the effects of different levels of stress on the pathogenesis of AD have not yet been elucidated. In the present study, we compared three types of psychological stress on mice with AD-like symptoms. Comparative analyses revealed that corticosterone levels (Fig. 1a) and the relative ratio of body weight loss (Fig. 1b) were more strongly affected in R-SDS-treated mice than in other mice. This result indicates a marked impact on relative body weight, particularly in SDS + Dfb + R-SDS-treated mice, suggesting a sustained effect of stressors on body weight changes. During the 1st week of psychological stress loading in the present study, urine corticosterone levels showed the highest increase in R-SDS-treated mice. After 2 weeks of psychological stress loading, urine corticosterone levels had decreased (Fig. 1a). This reduction may be due to a negative feedback mechanism that inhibits the activation of the HPA axis (16). As corticosterone may be an acute stress response marker, these results indicate that R-SDS was the strongest among the three psychological stressors examined, and also that the optimal time to examine the effects of psychological stress was during the 1st week of stress loading. Despite exposure to WS, mice exhibited higher corticosterone levels than those not exposed to any stress; however, this increase was not significant (Fig. 1a). The lower corticosterone levels in WS-treated mice may be due to an increased water intake diluting urine corticosterone levels. Although the total 24 h urine volume for all samples was recorded (17), we only collected samples 2 h after stress loading. Additionally, blood samples revealed corticosterone levels in response to noise stress during both the light and dark phases of the circadian cycle. The present study confirmed differential responsiveness to stressors in the acrophase and nadir of the circadian cycle, highlighting temporal variations in stress reactivity (18). Nevertheless, a urinary cortisol sample more accurately represents the production of cortisol over a specific duration, offering a more reflective measure of cortisol production than a single serum sample (19). Various perspectives exist regarding corticosterone as a stress marker among researchers, representing a limitation in our study.
Mechanical alloknesis scores, indicating sensitivity to mechanical stimuli, were markedly elevated in SDS + Dfb mice after the induction phase of AD (Fig. 2a and b). How-ever, no significant differences were observed between naive and SDS-treated mice. Additionally, we detected itch responses to mechanical stimuli in the naive group (Fig. 2a and b), suggesting that mechanically evoked itch is mediated by low-threshold mechanoreceptors (LTMs). Furthermore, itch appears to be constantly gated by a subpopulation of inhibitory neuropeptide Y (NPY) interneurons, which are also activated by LTM inputs under normal conditions (20). Moreover, we demonstrated that SDS+Dfb-treated mice exposed to psychological stress had significantly higher mechanical alloknesis scores than non-exposed SDS+Dfb-treated mice. Mechanical alloknesis refers to increased sensitivity to mechanical stimuli (Fig. 2c and d), which may result in itch. Previous studies demonstrated that changes in the NPY system play a crucial role in modulating the sensation of itch (21). This system is closely involved in the regulation of both the mechanical and histaminergic aspects of itch (22). Studies that examined NPY expression across the entire hypothalamus or within a specific region of the hypothalamic arcuate nucleus (ARC) consistently indicated the marked up-regulation of NPY transcription in response to various stressors. However, the precise nature and extent of this up-regulation appear to be associated with the specific type and duration of stress exposure (23).
An extensive analysis was conducted in the stress loading phase and revealed a correlation between urine corticosterone levels and alloknesis scores in SDS+Dfb+WS-treated mice at 0.07 g (Fig. 3a) and SDS+Dfb+R-SDS-treated mice at 0.07 g and 0.16 g (Fig. 3c). While no correlation was observed in SDS+Dfb+WS-treated mice at 0.16 g (Fig. 3a) or in SDS+Dfb+RS-treated mice (Fig. 3b), it is important to note that the strong correlation persisted between urine corticosterone levels and mechanical alloknesis scores in SDS+Dfb+WS-treated mice. This relationship underscores the complexity of the interplay between corticosterone levels and mechanical alloknesis responses under different treatment conditions during the stress loading phase.
Our previous histological findings revealed a higher number of intraepidermal nerve fibres in patients with AD than in healthy subjects (24, 25). Therefore, the higher density of intraepidermal nerve fibres may be at least partly responsible for itch sensitization. This may also be explained by our previous findings showing that film dressings inhibited itch hypersensitivity in a murine dry skin model (26). However, the present results suggest that the abundance of intraepidermal nerve fibres does not necessarily lead to an exaggerated perception of itch. Instead, a reduction and/or anomaly in intraepidermal nerve fibres appears to play a role in itch sensation (27). This study raises the question of how a reduction in intraepidermal nerve fibres is involved in itch, and one potential explanation is regional variations in the levels of some neuropeptides, including substance P and calcitonin gene-related peptide, both of which are involved in the itch sensation. Further research is needed to elucidate the specific mechanisms underlying the relationship between intraepidermal nerve fibres and itch perception.
In conclusion, the present study showed that psychological stress, particularly R-SDS, delayed improvements in dermatitis by exacerbating itch hypersensitivity in AD. The results obtained suggest that this model of psychological stress-induced mechanical alloknesis is useful for investigating the mechanisms underlying stress-induced itch hypersensitivity and will contribute to the development of potential therapeutic strategies that mitigate psychological stress-related effects.
ACKNOWLEDGEMENTS
IRB approval status: Animal experiments were performed according to the protocols of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Experimental protocols were approved by the Ethical Committee of Research Animal Use of Juntendo University (Approval No. 2023088).
REFERENCES
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015; 66: 8–16.
- Lifschitz C. The impact of atopic dermatitis on quality of life. Ann Nutr Metab 2015; 66: 34–40.
- Kawakami Y, Yumoto K, Kawakami T. An improved mouse model of atopic dermatitis and suppression of skin lesions by an inhibitor of Tec family kinases. Allergol Int 2007; 56: 403–409.
- Napolitano M, di Vico F, Ruggiero A, Fabbrocini G, Patruno C. The hidden sentinel of the skin: an overview on the role of interleukin-13 in atopic dermatitis. Front Med (Lausanne) 2023; 10: 1165098.
- Matucci A, Vivarelli E, Nencini F, Maggi E, Vultaggio A. Strategies targeting type 2 inflammation: from monoclonal antibodies to JAK-inhibitors. Biomedicines 2021; 9: 1497.
- Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci 2017; 18: 2131.
- Alexopoulos A, Chrousos GP. Stress-related skin disorders. Rev Endocr Metab Disord 2016; 17: 295–304.
- Nie YM, Ishii I, Yamamoto K, Orito K, Matsuda H. Real-time scratching behavior quantification system for laboratory mice using high-speed vision. J Real Time Image Process 2009; 4: 181–190.
- Toyama S, Moniaga CS, Nakae S, Kurosawa M, Ogawa H, Tominaga M, et al. Regulatory T cells exhibit interleukin-33-dependent migratory behavior during skin barrier disruption. Int J Mol Sci 2021; 22: 7443.
- Zhao QF, Koyama S, Yoshihara N, Takagi A, Komiyama E, Wada A, et al. The alopecia areata phenotype is induced by the water avoidance stress test in cchcr1-deficient mice. Biomedicines 2021; 9: 840.
- Lee EH, Park JY, Kwon HJ, Han PL. Repeated exposure with short-term behavioral stress resolves pre-existing stress-induced depressive-like behavior in mice. Nat Commun 2021; 12: 6682.
- Higashida S, Nagai H, Nakayama K, Shinohara R, Taniguchi M, Nagai M, et al. Repeated social defeat stress impairs attentional set shifting irrespective of social avoidance and increases female preference associated with heightened anxiety. Sci Rep 2018; 8: 10454.
- Komiya E, Tominaga M, Hatano R, Kamikubo Y, Toyama S, Sakairi H, et al. Peripheral endomorphins drive mechanical alloknesis under the enzymatic control of CD26/DPPIV. J Allergy Clin Immunol 2022; 149: 1085–1096.
- Arndt J, Smith N, Tausk F. Stress and atopic dermatitis. Curr Allergy Asthma Rep 2008; 8: 312–317.
- Morren MA, Przybilla B, Bamelis M, Heykants B, Reynaers A, Degreef H. Atopic dermatitis: triggering factors. J Am Acad Dermatol 1994; 31: 467–473.
- Mikulska J, Juszczyk G, Gawronska-Grzywacz M, Herbet M. HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci 2021; 11: 1298.
- Lapointe JM, Snyder PA, Reagan WJ. Evaluation of urinary corticosterone as a biomarker of stress in rats using fenitrothion as a chemical stressor. J Immunotoxicol 2016; 13: 386–392.
- Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL. Diurnal variation in the responsiveness of the hypothalamic–pituitary–adrenal axis of the male rat to noise stress. J Neuroendocrinol 2006; 18: 526–533.
- Turpeinen U, Hamalainen E. Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab 2013; 27: 795–801.
- Andersen HH, Akiyama T, Nattkemper LA, van Laarhoven A, Elberling J, Yosipovitch G, et al. Alloknesis and hyperknesis-mechanisms, assessment methodology, and clinical implications of itch sensitization. Pain 2018; 159: 1185–1197.
- Jakobsson JET, Ma H, Lagerstrom MC. Neuropeptide Y in itch regulation. Neuropeptides 2019; 78: 101976.
- Gao T, Ma H, Xu B, Bergman J, Larhammar D, Lagerstrom MC. The neuropeptide Y system regulates both mechanical and histaminergic itch. J Invest Dermatol 2018; 138: 2405–2411.
- Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides 2016; 55: 99–109.
- Tominaga M, Takamori K. An update on peripheral mechanisms and treatments of itch. Biol Pharm Bull 2013; 36: 1241–1247.
- Tominaga M, Takamori K. Peripheral itch sensitization in atopic dermatitis. Allergol Int 2022; 71: 265–277.
- Iwanaga T, Tominaga M, Hirata Y, Matsuda H, Shimanuki T, Ogawa H, et al. Effects of film dressings on itch hypersensitivity using murine dry skin models. Acta Derm Venereol 2018; 98: 902–903.
- Hashimoto T, Yosipovitch G. Itchy body: topographical difference of itch and scratching and C nerve fibres. Exp Dermatol 2019; 28: 1385–1389.