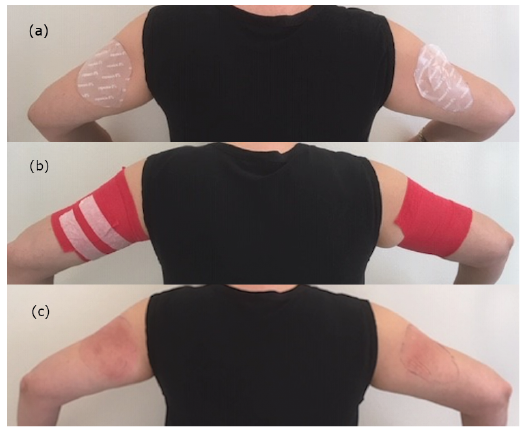
Fig. 1. Areas with touch-evoked itch were identified and (a) patches were cut to cover the areas and (b) applied for 1 h under bandage occlusion. (c) Subsequent erythema of the treated areas of skin.
1National Allergy Research Centre, and 2Department of Dermatology and Allergy, Herlev and Gentofte Hospital, University of Copenhagen, Gentofte Hospitalsvej 20A, 1st floor, DK-2900 Hellerup, Denmark. E-mail: stine.hoffmann@regionh.dk
Accepted Nov 4, 2020; Epub ahead of print Nov 10, 2020
Granulomas as an adverse effect following subcutaneous immunotherapy (SCIT) or immunization with other aluminium-adsorbed vaccines has been described since the 1980s (1–3). Despite the overall benign nature of aluminium granulomas, they can cause aggravating itch, and lead to reduced quality of life and discontinuation of both common childhood vaccination programmes and SCIT (4). An effective treatment is yet to be established. We present here a case of, to our knowledge, the first treatment attempt for itching granulomas with capsaicin-patches, which had a successful outcome.
A 48-year old female was referred to our department with a medical history of multiple IgE allergies, rhinoconjunctivitis, asthma and eczema.
During childhood she had received treatment with multiple SCIT injections against birch and grass pollen on both lower arms. SCIT was discontinued due to multiple local subcutaneous nodules causing aggravating itch. All former granulomas had disappeared, but her lower arms were still covered with scars and hyperpigmentation following years of scratching. She was patch-tested in 2015 with aluminium chloride hexahydrate 2% pet. and an empty Finn Chamber (Epitest, Tuusula, Finland). Patch-tests were applied for 2 days and read on days 2, 4 and 7 according to recommendations. The test results were negative.
Due to severe respiratory allergy, SCIT injections were resumed, this time on both upper arms. Again, the patient developed local reactions with small itching subcutaneous nodules causing scratch marks and hyperpigmentation, and finally SCIT treatment was terminated, and sublingual immunotherapy was initiated, but the multiple nodules on both upper arms were still severely itching. No flare of itch was seen in the former granulomas on the lower arms. Treatment with antihistamine and topical corticosteroids were not effective. Intra-granuloma injections with triamcinolone acetonide had some effect, but this treatment was not feasible due to the large number of granulomas. Topical capsaicin cream 0.025% was applied 2–4 times daily for 5 weeks and had a positive, but not sufficiently large, effect.
In April 2018, the patient received the first off-label treatment with a Qutenza® 8% capsaicin patch. On her right upper arm, the area with touch-evoked itch (alloknesis) was identified and marked with a pen. The patch was cut with a pair of scissors to cover the area and applied for 1 h under bandage occlusion. After removal, the area was wiped with a cleansing gel and washed with water (Fig. 1).

Fig. 1. Areas with touch-evoked itch were identified and (a) patches were cut to cover the areas and (b) applied for 1 h under bandage occlusion. (c) Subsequent erythema of the treated areas of skin.
Mean daily itch severity was evaluated using a visual analogue scale (VAS) with 0 as “no itch” and 10 as “worst imaginable itch” (Fig. 2). Before initiation of Qutenza® treatment, the itch was graded as VAS 8 on both arms. Six weeks after the first treatment, itch was unaffected, i.e. VAS 8, on the untreated arm and was reduced to VAS 4 on the treated arm. VAS 4 is described by the patient as several daily disturbances by itch, where scratching or pinching is necessary.
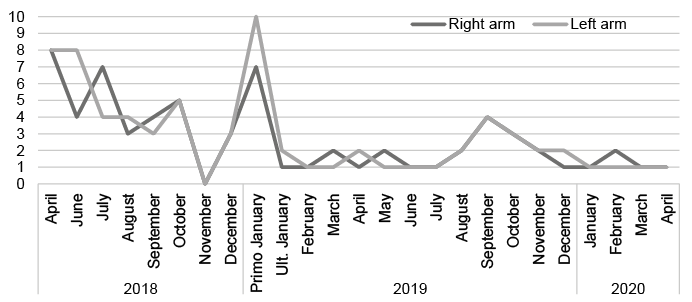
Fig. 2. Mean daily itch score on a 0–10 visual analogue scale (VAS).
Due to the perceived effectiveness of the Qutenza® patch lasting 2–3 months, treatment was repeated on both arms every third month, with VAS scores gradually reducing. Except for erythema and a burning sensation during treatment, and increased sensitivity to touch, heat and sun exposure, normalizing within 3–4 days, no other adverse effects were seen.
Qutenza® (Grünenthal GmbH, Achen, Germany) is a capsaicin 8% topical patch mainly used for the treatment of neuropathic pain (5, 6). Recently the use of Qutenza® patches has been expanded to include neuropathic itch (7). The patches deliver a high concentration of synthetic capsaicin, which works as a transient receptor potential vanilloid-1 (TRPV-1) agonist. When applying the patch, the local cutaneous nociceptors are activated, leading to pain and erythema, and following exposure there is a desensitization, which causes the itch-relieving effect. Side-effects of Qutenza® are mainly application-site erythema and pain, which can be quite significant and necessitate use of pain-relieving medication during the application.
Itching subcutaneous granulomas are mostly seen in children; 1% develop granulomas following vaccination with aluminium-adsorbed vaccines (8). The clinical presentation of subcutaneous granulomas is usually a firm nodule on the injection site, with associated pruritus. Hypertrichosis and hyperpigmentation are also common (4, 8).
Of all children with vaccination granulomas, 95% become hypersensitized against aluminium (3).
Aluminium contact allergy has also been described in older children and adults undergoing SCIT (1, 2). The current patient was patch-tested in 2015, with a negative result. However, this was prior to resumption of her SCIT treatment, and the granulomas on her lower arms from SCIT treatments in childhood were asymptomatic. This correlates with the fact that aluminium contact allergy and the granulomas are known to diminish over time (9, 10).
Because the current patient was still symptomatic, a patch-test was performed again in September 2020 with aluminium chloride hexahydrate 2% pet. and an empty metallic Finn Chamber (Epitest, Tuusula, Finland). The test was once again negative; however, she developed severe itch on her palms and feet and an intense flare-up of granuloma itch. It has been suggested that older children and adults with suspected aluminium allergy but a negative patch-test to aluminium chloride hexahydrate 2% pet., should be tested with 10% pet. instead, in order to avoid false negative results (11). Because the current patient experienced a severe flare up in itch during patch-testing, indicating a systemic reaction, it was decided not to repeat a patch-test with aluminium chloride hexahydrate 10%. Infections and aluminium-containing products are reported to cause aggravated itch (4). In the current patient the granulomas were particularly itchy in the beginning of January 2019 and September 2019, with daily VAS scores up to 8–10, when she went through some work-related challenges.
There are limited treatment options for achieving relief of itch in patients with itching subcutaneous granulomas. In the current patient, Qutenza® treatment significantly reduced the itch in both intensity and area, respectively. Pretreatment with lidocaine (4%) or lidocaine (2.5%)/prilocaine (2.5%) may be used prior to the application, as suggested by the manufacturer, to sooth the skin prior to applying the patches (7). This could lead to more patients with granulomas undergoing this treatment. If capsaicin treatment in children is considered, capsaicin cream 0.025%, applied twice daily for 6 weeks, could be a safer regimen than capsaicin patches. Treatment of children is delicate, and applying an 8% capsaicin patch should be considered with caution until further studies on adults have been completed.
Funding. The Danish Environmental Protection Agency; a grant to the National Allergy Research Centre.
The authors have no conflict of interest to declare.