QUIZ SECTION
Multiple Ulcerative Nodules on the Neck and Trunk: A Quiz
Yu NISHIMURA, Hiroyuki GOTO, Junko SOWA-OSAKO and Daisuke TSURUTA
Department of Dermatology, Graduate School of Medicine, Osaka Metropolitan University, 1-4-3, Asahimachi, Abeno-ku, Osaka 545-8585, Japan. *E-mail: h.gotou.19850820@gmail.com
Citation: Acta Derm Venereol 2023; 103: adv18695. DOI https://doi.org/10.2340/actadv.v103.18695.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Published: Dec 13, 2023
A 59-year-old man was referred to our department with multiple ulcerative nodules on his neck and trunk. He had been diagnosed as having atopic dermatitis when he was 20 years old and had received treatment with a topical corticosteroid ointment. The nodules had appeared and enlarged 3 months before the current examination. Physical examination revealed ulcerative nodules on his neck, abdomen and thigh, approximately 1–2 cm in size (Fig. 1a, b). Positron emission tomography-computed tomography (PET-CT) revealed accumulation of fluorodeoxyglucose only in the cutaneous nodules without lymph node involvement. Haematoxylin and eosin (H&E) staining of a histopathological specimen from the neck lesion revealed ulcerative epidermis and dense infiltration of large atypical lymphoid cells (Fig. 1c, d). Immunohistochemical staining showed that those tumour cells were positive for CD3, CD4, CD30, and negative for CD20 (Fig. 1e). The Ki-67 positive ratio was approximately 40% in those atypical lymphoid cells (Fig. 1f). Examination of T cell receptor rearrangement (β chain Cβ1) was positive. Serum levels of lactate dehydrogenase (287 U/L, normal range 124–222 U/L) and soluble IL-2 receptor (550 U/mL, normal range 204–587 U/mL) were normal, and serum levels of thymus and activation-regulated chemokine (5,626 pg/mL, normal range < 450 pg/mL) and total IgE (25,000 > IU/mL, normal range < 170 IU/mL) were increased.
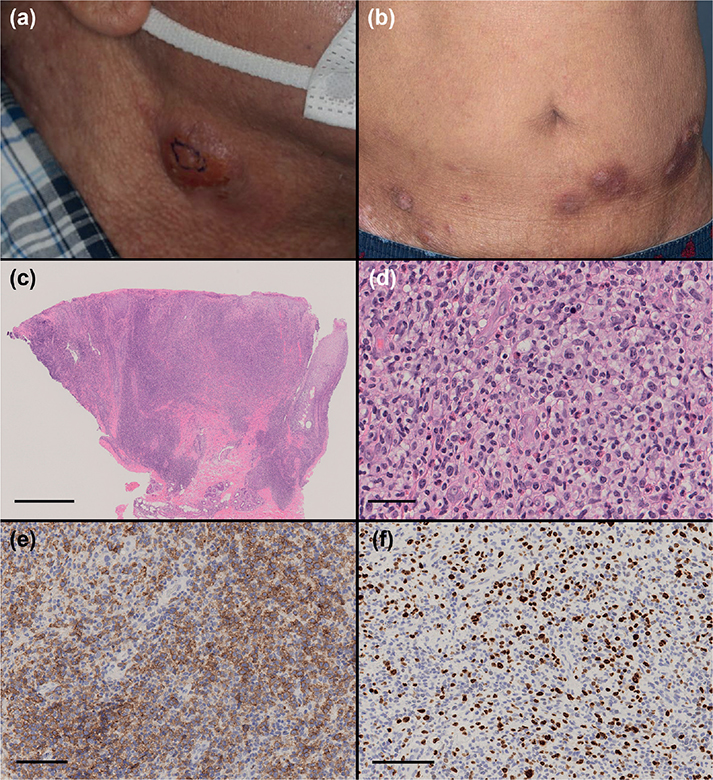
Fig. 1. (a) Physical examination revealed nodules on the neck, 1–2 cm in size. (b) Ulcerative nodules, plaques and erythema were observed on the abdomen and thigh. (c) Histopathological examination revealed dense infiltration of tumour cells from the epidermis to deep dermis (haematoxylin and eosin (H&E) staining, bar = 1 mm). (d) The tumour cells were composed of large atypical lymphoid cells (H&E staining, bar = 100 μm). (e) The atypical cells were positive for CD30 (CD30 staining, bar = 100 μm). (f) The Ki-67-positive ratio was approximately 40% (Ki-67 staining, bar = 100 μm).
What is your diagnosis?
Differential diagnosis 1: Mycosis fungoides with large cell transformation
Differential diagnosis 2: Primary cutaneous anaplastic large cell lymphoma
Differential diagnosis 3: Lymphomatoid papulosis
See next page for answer.
ANSWERS TO QUIZ
Multiple Ulcerative Nodules on the Neck and Trunk: A Commentary
Diagnosis: Mycosis fungoides with large cell transformation
An additional 3 skin biopsies were performed from the plaques and erythema on his trunk. Two of those histopathological specimens revealed that atypical lymphoid cells had infiltrated the epidermis and superficial dermis with irregular fibrosis of the papillary dermis (Fig. 2a). Large atypical cells, such as those in the first biopsied specimen, were not observed in those specimens. The infiltrating cells were positive for CD3 and CD4 and negative for CD20. Some of the infiltrating cells were positive for CD30, and the Ki-67-positive ratio was approximately 20% (Fig. 2b). Because the specimens showed the existence of mycosis fungoides, we finally made a diagnosis of mycosis fungoides with large cell transformation (MF-LCT). The other histopathological specimen showed acanthosis, spongiosis and pseudo-Pautrier microabscess in the epidermis and infiltration of normal lymphocytes and eosinophils in the superficial dermis, namely subacute eczema (Fig. 2c). Therefore, we considered that the patient had atopic dermatitis. The patient underwent combination therapy of bexarotene and narrow-band ultraviolet B (UVB). Two weeks after starting treatment, the plaques and erythemas were improved, and the modified Severity-Weighted Assessment Tool score decreased from 11 points to 7.5 points (Fig. 2d).
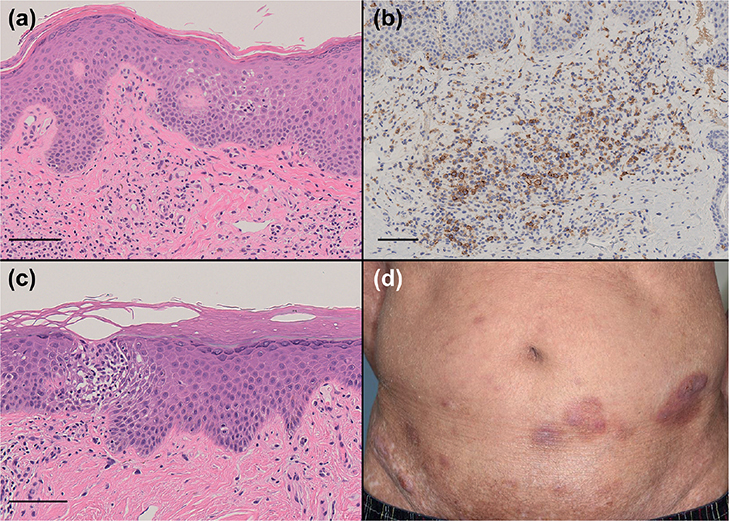
Fig. 2. (a) The histopathological specimen from the plaque showed infiltration of atypical lymphocytes in the epidermis and dermis with irregular papillary dermal fibrosis (haematoxylin and eosin (H&E) staining, bar = 100 μm). (b) Some of the infiltrating lymphocytes in the dermis were positive for CD30 (CD30 staining, bar = 100 μm). (c) The histopathological specimen from the erythema showed acanthosis, spongiosis, pseudo-Pautrier microabscess in the epidermis and infiltration of non-atypical lymphocytes in the epidermis and superficial dermis, namely findings of eczema (H&E staining, bar = 100 μm). (d) After starting treatment with bexarotene and narrow-band UVB, the plaques and erythema were improved.
MF-LCT is defined as MF in which more than 25% of the tumour cells are large cells (more than 4-times larger than the size of normal lymphocytes) (1). Those large cells can be both CD30-positive and CD30-negative. CD30-positive lymph proliferative disease includes lymphomatoid papulosis and anaplastic large cell lymphoma (ALCL) as skin diseases (2). Cutaneous ALCL (cALCL) is defined as a disease in which more than 75% of the tumour cells are positive for CD30 (1). In the current case, the tumour included dense infiltration of CD30-positive and T lymphocyte marker-positive large atypical cells in the epidermis to dermis. Based on clinical findings such as an ulcerative tumour and non-self-healing course, we ruled out the possibility of lymphomatoid papulosis and considered the possibility of MF-LCT and cALCL as differential diagnoses. For making a diagnosis of cALCL, the patient should not have clinical evidence or a history of MF (1). Therefore, we needed to perform additional skin biopsies to demonstrate the existence of MF. Making a diagnosis of MF is often difficult, particularly in patients with atopic dermatitis (3). In the current case, although some specimens included infiltration of atypical lymphoid cells in the epidermis and dermis with irregular papillary dermal fibrosis, the other specimen showed findings of eczema. Therefore, multiple biopsies from erythemas and papules are important to make a correct diagnosis of MF, especially for differentiating between MF-LCT and cALCL.
LCT is considered to be a poor prognostic factor in MF, and the median overall survival period in such patients has been reported to be only 19.4 months (4). On the other hand, cALCL has a relatively preferable prognosis, and the 5-year disease-specific survival rates in such patients has been reported to be 93% in T1 and T2 and 77% in T3 (5). With regard to treatment, cALCL is often treated with local therapy, such as surgical resection and radiation therapy, but MF-LCT usually needs systemic therapy including retinoid, interferon and chemotherapy (4, 5). Therefore, differentiating between MF-LCT and cALCL is important, and careful physical examination and repeated skin biopsies are necessary.
REFERENCES
- Kadin ME, Hughey LC, Wood GS. Large-cell transformation of mycosis fungoides-differential diagnosis with implications for clinical management: a consensus statement of the US Cutaneous Lymphoma Consortium. J Am Acad Dermatol 2014; 70: 374–376.
- Elder DE MD, Scolyer RA, Willemze R. WHO classification of skin tumors. Lyon, France: International Agency for Research on Cancer; 2018.
- Roenneberg S, Braun SA, Garzorz-Stark N, Stark SP, Muresan AM, Schmidle P, et al. Histology-based classifier to distinguish early mycosis fungoides from atopic dermatitis. J Eur Acad Dermatol Venereol 2023; 37: 2284–2292.
- Diamandidou E, Colome-Grimmer M, Fayad L, Duvic M, Kurzrock R. Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood 1998; 92: 1150–1159.
- Benner MF, Willemze R. Applicability and prognostic value of the new TNM classification system in 135 patients with primary cutaneous anaplastic large cell lymphoma. Arch Dermatol 2009; 145: 1399–1404.