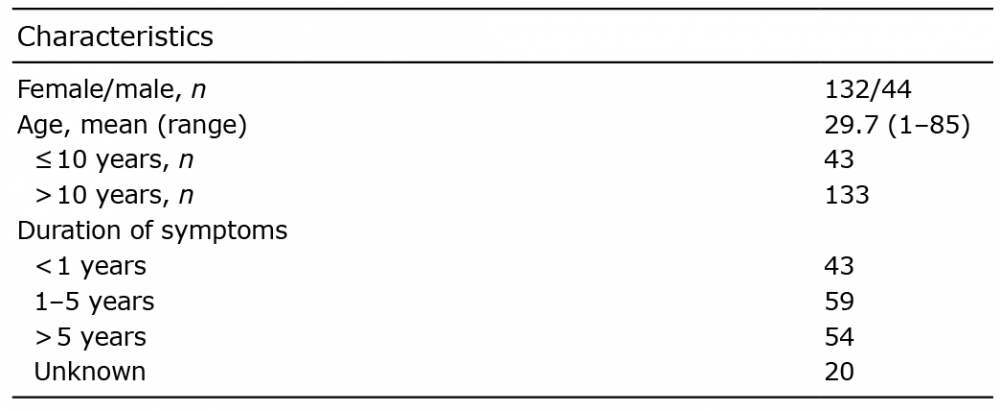
Table I. Characteristics of the study population (n = 176)
1Faculty of Medicine, 2Infrastructure for Population Studies, Medical Faculty, and 3Department of Dermatology and Medical Research Center Oulu, PEDEGO Research Unit, University of Oulu, Oulu University Hospital, Oulu, Finland. E-mail: kaisa.tasanen@oulu.fi
Accepted Jan 27, 2020; Epub ahead of print Jan 30, 2020
Acta Derm Venereol 2020; 100: adv00063
Alopecia areata (AA) is a globally significant disease. Several studies have estimated the prevalence of AA worldwide to be 1–2% (1). It is an autoimmune-mediated disease, which is not linked to age, sex or ethnicity, that directly causes non-scarring hair loss (1–4). In addition to the scalp, AA can cause hair loss in other areas of the body and can also cause nail dystrophy (3). Its manifestation ranges from patchy hair loss to AA universalis (total loss of all scalp and body hair) (4); however, the severity of AA cannot be defined by its histopathology (5). The prognosis of AA and its response to treatment are difficult to predict because the course of disease is highly variable (5).
The pathogenesis of AA is unknown. Several studies have suggested that both genetic and environmental factors, such as hormonal instability and stress, affect its aetiology (3, 6). As is often the case with autoimmune diseases, AA has been associated with DR and DQ regions of the human leukocyte antigen (HLA), which affects antigen presentation (7).
A diagnosis of AA has been associated with an increased risk of one or more other autoimmune diseases. Such comorbid conditions include systemic autoimmune disorders, such as ulcerative colitis, type 1 diabetes, thyroid diseases, coeliac disease and rheumatoid arthritis, and dermatological autoimmune conditions, including vitiligo, lupus erythematosus and scleroderma (3, 8). To date, most of the data available on comorbidities of AA have been drawn from Asian populations. The aim of this study was therefore to evaluate the comorbidities of AA in the predominantly Caucasian population served by the Northern Ostrobothnia Hospital District (NOHD), Finland.
Patient records were retrieved following a search of the Oulu University Hospital (OUH) database based on a diagnosis of AA between 1987 and 2016, defined by the presence of International Classification of Diseases (ICD) codes 7040 in ICD-9 and L63 in ICD-10. All of the retrieved records were checked by the authors for demographics and pre-selected comorbidities. Statistical analyses were performed using IMB SPSS statistics and Stata version 13 (Stata Statistical Software: Release 13, College Station, TX, USA: StataCorp LP, 2013) software. The results are presented as proportions and means. The study methods were approved by the medical director of OUH. As the study was based on a retrospective review of records, the agreement of the ethics committee was not required.
The database search returned records of 176 patients diagnosed with AA in OUH during the study period. There were 132 females (75%) and 44 males (25%), a female/male ratio of 3:1. The mean age of participants was 29.7 years (median 28.0) and 43 participants were aged ≤10 years (Table I). More than 30% (n = 54) of patients had had symptoms of AA for longer than 5 years. A total of 107 (60.8%) patients had at least one comorbid disease. All the investigated comorbidities are shown in Fig. 1. The most common comorbidities were eczema, which was present in 48 patients (27.3%), thyroid diseases in 30 (17.0%) patients and cardiovascular diseases in 23 (13.1%) patients.

Table I. Characteristics of the study population (n = 176)
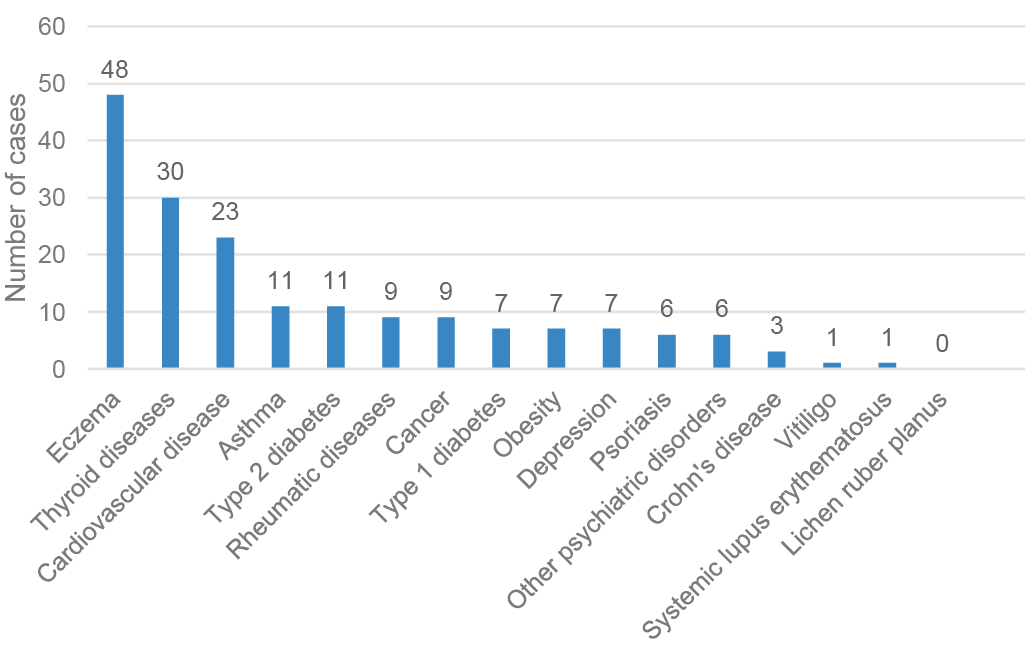
Fig. 1. Comorbid diseases in patients with diagnosed alopecia areata in Northern Finland between 1987 and 2016.
More than 60% of the patients with AA in this study had one or more comorbid diseases, a finding that is comparable with those of previous studies, which found that most participants had had at least one comorbid disease (9). With a mean age of 29.7 years and approximately one-quarter (24.4%, n = 43) being younger than 10 years, our patient population aligns with those of previous studies (4, 10). However, in our study the female:male ratio was 3:1, which contrasts with previous findings that the prevalence of AA does not differ between the sexes (1, 3, 4). This finding may be at least partially explained by differences between various cultures in terms of which sex is more likely to seek healthcare. The duration of AA depends on the severity of its manifestation and is difficult to predict, due to the great diversity of its course (11). In the current study, more than one-third (n = 54) of cases had had AA symptoms for over 5 years. This is slightly longer than might be expected according to previous reports that 30–50% of AA cases resolve within one year (11).
Several studies support an underlying autoimmune mechanism for the pathology of AA, but with genetic and environmental factors triggering its onset (3).
Petukhova et al. (7) noticed that AA is associated with the presence of alleles that affect both innate and adaptive immunity. Several genomic regions, including the T-cell differentiation and maintenance regulatory genes, have been linked with AA (3, 7). Furthermore, other studies have demonstrated that AA is associated with increased risks of other autoimmune diseases, and of several atopic, cardiovascular, gastrointestinal, connective tissue, dermatological, haematological, and thyroid diseases, as well as psychiatric disorders (2, 4, 8). As shown in Fig. 1, the findings of the current study support these associations.
Atopic eczema was found in 27% of our patients with AA, a finding that aligns with those of several previous studies that have reported a clear association between atopic eczema and AA, including a US study that found atopy in one-third of patients with AA. However, it is of note that, in the US study, allergic rhinitis, asthma and dermatitis were reported together (12). Conversely, it has been demonstrated that the patients with pre-existing atopic dermatitis had a 1.7-fold increased risk of subsequent AA (4). The background mechanisms of this association are not fully understood, but it has been suggested that the pathogenesis of AA has features of both atopy and autoimmune-based skin disease (4). The observed prevalence of thyroid diseases in the current study was 17%, which is slightly higher than the 14% found in a recent meta-analysis (8). A significant association has been reported between AA and various thyroid diseases, especially hypothyroidism (8, 13). Moreover, it has been shown that patients with hypothyroidism have a 3-fold increased risk of developing AA (4).
AA causes significant cosmetic disadvantage and has negative effects on patients’ quality of life (3). Patients with AA are particularly predisposed to psychiatric disorders (8): a Spanish study found that as many as 66% of patients with AA have at least one psychiatric condition, most commonly adjustment disorder, generalized anxiety or depression (14). Few psychiatric comorbidities were found in the current study population. It is possible that, in the patients in the current study, who have typically had only a single dermatological consultation, psychiatric disorders may have been undiagnosed in the hospital setting. However, when treating patients with AA, it is important to consider their psychological wellbeing because of the known effect of AA on quality of life (15).
The main strength of this study is its comprehensive population; the analysis included all patients diagnosed with AA at OUH, which has the only department of dermatology in the NOHD. However, a limitation of this study was the relatively small study population, due to the rarity of AA in Finland. Nevertheless, the findings of this study of the Caucasian population are generally comparable with previous findings in Asian populations, except for our finding of a significant female predominance of AA.