
Table I. Sociodemographic and disease-related characteristics of the study cohort
1Department of Dermatology and Phlebology, Vivantes Klinikum im Friedrichshain, Berlin, 2Mannheim Institute of Public Health, Social and Preventive Medicine, Medical Faculty Mannheim, 3Department of Dermatology, Venereology and Allergology, University Medical Center Mannheim, Heidelberg University, Mannheim, 4Department of Dermatology, Venereology und Allergology, Charite? University Medicine Berlin, 5Department of Dermatology and Allergology, Vivantes Klinikum Spandau, 6Department of Dermatology and Venereology, Vivantes Klinikum Neuko?lln, Berlin, and 7Skin Cancer Unit, German Cancer Research Center (DKFZ), Heidelberg, Germany
Treatment paradigms for advanced melanoma have changed fundamentally over recent years. A discrete choice experiment was performed to explore patient preferences regarding outcome (overall response rate, 2-year survival rate, progression-free survival, time to response, type of adverse events, probability of adverse event-related treatment discontinuation) and process attributes (frequency and route of administration, frequency of consultations) of modern treatments for melanoma. Mean preferences of 150 patients with melanoma stage IIC–IV were highest for overall response rate (relative importance score (RIS) 26.8) and 2-year survival (RIS 21.6), followed by type of adverse events (RIS 11.7) and probability of adverse event-related treatment discontinuation (RIS 9.2). Interest in overall response rate and 2-year survival declined with increasing age, whereas process attributes gained importance. Participants who had experienced treatment with immune checkpoint inhibitors valued overall response rate more highly and worried less about the type of adverse events. In conclusion, patients with advanced melanoma consider efficacy of treatment options most important, followed by safety, but preferences vary with individual and disease-related characteristics.
Key words: patient preferences; conjoint analysis; melanoma; targeted therapy; BRAF inhibitors; immune checkpoint inhibitors.
Accepted Feb 10, 2020; Epub ahead of print Feb 14, 2020
Acta Derm Venereol 2020; 100: adv00083.
Corr: Wiebke K. Ludwig-Peitsch, Department of Dermatology and Phlebology, Vivantes Klinikum im Friedrichshain, Landsberger Allee 49, DE-10249 Berlin, Germany. E-mail: wiebke.ludwig-peitsch@vivantes.de
Treatment paradigms for advanced melanoma have changed substantially during recent years. For patients with BRAF-mutated melanoma in particular, several first-line palliative or adjuvant treatments may be feasible, which differ considerably in terms of efficacy, safety profile and mode of application. A discrete choice experiment was performed to explore the preferences of 150 patients with advanced melanoma regarding outcome and process attributes of modern treatments in 5 German dermato-oncology departments. Mean preferences were highest for overall response and 2-year survival, followed by safety. However, patient preferences varied significantly, depending on their socio-demographic characteristics and treatment experience. Assessing and considering these preferences is fundamental for patient-centred care.
The incidence of malignant melanoma is constantly increasing worldwide (1), and has quintupled in Germany since the 1970s (2). Melanoma is currently the fifth most common tumour entity among men and women in Germany, causing 4.5% of all cases of cancer (2). If diagnosed at an early stage, the prognosis for patients with melanoma is promising, with 10-year survival rates up to 98% (3). However, 10-year survival for high-risk primary melanoma in stage IIC is only 75%, and survival rates in stage III with regional metastases range between 24% and 88% and decline further for stage IV (3).
Treatment paradigms for advanced melanoma have been revolutionized in recent years, following the approval in 2011 of the first immune checkpoint inhibitor, ipilimumab, targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (4). Since then, the spectrum of immune checkpoint inhibitors has been expanded by the programmed cell death 1 protein (PD-1) antibodies nivolumab and pembrolizumab (5–8), and by approval of nivolumab in combination with ipilimumab (9, 10). In addition, targeted therapies inhibiting the serine/threonine-protein kinase B-Raf and the mitogen-activated protein kinase-kinase (MEK), i.e. dabrafenib and trametinib, vemurafenib and cobimetinib and, most recently, encorafenib plus binimetinib, were introduced for treatment of advanced BRAF-mutated melanoma (11–16). Another treatment option for unresectable melanoma in stage III and IV M1a is talimogene laherparepvec (T-VEC), a genetically engineered oncolytic virus that is injected intralesionally into the tumour tissue (17). The landscape of melanoma therapy is evolving rapidly, and a plethora of novel targeted therapies, immune checkpoint inhibitors, combination therapies and neoadjuvant treatment regimens are under investigation (18, 19).
Immune checkpoint inhibitors and combined BRAF- and MEK-targeted therapies have improved survival outcomes substantially. For example, recently published long-term data from clinical trials show a 4-year overall survival rate of 53% for nivolumab combined with ipilimumab (9) and a 5-year overall survival rate of 34% for the combination of dabrafenib and trametinib (13). First developed for palliative melanoma therapy, dabrafenib plus trametinib, nivolumab and pembrolizumab were more recently also approved for adjuvant treatment of melanoma stage III and/or IV (20–22), which led to a profound renewal of adjuvant treatment regimens.
For patients with BRAF-mutated melanoma, several options may be feasible as first-line palliative or adjuvant treatment (23). In the palliative setting, patients and physicians have the choice of 3 different BRAF- and MEK-inhibitor combinations, 2 PD-1 antibodies as monotherapy, nivolumab combined with ipilimumab and, sometimes, T-VEC. These treatment approaches differ considerably with regard to efficacy outcomes, adverse event (AE) profiles and modes of application. For example, the combination of nivolumab and ipilimumab offers a higher objective response rate, longer progression-free survival (PFS) and longer overall survival (OS) than nivolumab monotherapy, but also bears a significantly higher risk of grade 3/4 AE (9).
With an increasing number of treatment options, the process of shared decision-making becomes increasingly complex. In addition to objective patient- and tumour-related criteria, it is extremely important to integrate patients’ individual values, demands, fears and preferences into treatment decisions. The responsibility of physicians to consider patients’ treatment goals was emphasized recently in a consensus guideline on patient-physician communication produced by the American Society of Oncology (24). A stronger orientation towards patients’ needs in oncological care was also stated in the National Cancer Plan of the German Federal Ministry of Health (25).
Preferences regarding cancer treatment can be assessed using a plethora of methods, including ranking or rating scales, visual analogue scales, standard gamble, time trade-off, multi-attribute utility instruments and discrete choice experiments (DCEs) (26). DCEs, also known as choice-based conjoint analysis, have attained increasing popularity in healthcare research within the last few decades, both in the fields of dermatology (27) and oncology (28). This method provides the advantage of realistically reflecting clinical decision-making. In DCEs participants are confronted with hypothetical scenarios that are defined by a number of characteristics (attributes) and differ in the corresponding attribute levels. Two or more scenarios are combined into a choice set and participants are asked to choose their preferred option. By using regression techniques to analyse multiple choice decisions, individual preferences and the relative influence of each attribute on choice behaviour can be determined (29).
In the past, research on treatment preferences of patients with melanoma focused on adjuvant interferon-α therapy (30–32). More recently, 3 research groups have performed DCEs to assess patient preferences for BRAF- and MEK-inhibitors compared with immune checkpoint inhibitors (33–36). All of these studies were initiated and/or supported by the manufacturers of these medications, i.e. Merck (33, 34), Bristol-Myers Squipp (35) and Novartis (36).
The aims of the current study were to investigate the preferences of German patients with melanoma regarding outcome and process attributes of modern systemic melanoma treatments, including BRAF- and MEK-inhibitors, immune checkpoint inhibitors, and T-VEC, in a multicentric DCE, independent of the pharmaceutical industry, and to determine the impact of patient-, tumour- and treatment-related characteristics on these preferences.
Study cohort
Participants were recruited from 5 centres specialized in dermato-oncology in Germany. Inclusion criteria were: physician-confirmed diagnosis of melanoma AJCC 2017 stage IIC–IV (3); age ≥18 years; ability to provide written informed consent; and German language skills. All patients fulfilling these criteria were eligible for participation, i.e. patients both with and without current tumour burden and patients both with and without previous or current adjuvant or palliative systemic melanoma treatment could be included. Critically ill patients, who did not feel able to complete the survey at the time, were invited to participate at a later date. Data collection was accomplished between 1 August 2017 and 28 February 2019. The study was approved by the ethics committee of the Faculty of Medicine of Charité University Medicine Berlin (EA4/110/17) and conducted according to the principles of the Declaration of Helsinki.
Data collection
Inpatients and outpatients visiting skin cancer clinics for melanoma treatment or follow-up examinations were asked to participate while waiting for their consultation. After providing written informed consent, they received access to a computer-based survey. Support in operating the computer was provided, if needed. Within the survey, participants were confronted with a DCE. In addition, they were asked about sociodemographic and disease-related characteristics (age, sex, marital status, education, employment status, time since diagnosis, previous and current melanoma treatments). Information on AJCC stage, tumour burden and the treatment course was extracted from the medical records.
Discrete choice experiment
The questionnaire including the DCE was generated using Sawtooth Software (Lighthouse Studio version 9.3.1, Provo, UT, USA). For creation of discrete choice scenarios, modern systemic medications currently approved for treatment of advanced melanoma in Germany were decomposed into 6 outcome attributes (overall response rate (ORR), defined as partial or complete response; 2-year survival rate; progression-free survival (PFS); time to response; type of adverse events (AE); probability of AE-related treatment discontinuation) and 3 process attributes (route of administration; frequency of administration; frequency of consultations). Four levels, reflecting the range of plausible values for systemic therapies including immune checkpoint inhibitors (PD-1 and/or anti-CTLA-4 antibodies), targeted therapies (BRAF- and MEK-inhibitors) and T-VEC, were assigned to each attribute. Attributes and attribute levels (Table SI) were selected based on extensive literature research, including clinical trials (4, 5, 7, 10–12, 14–17), reviews and prescribing information. A cost attribute was not included, as German health insurance covers the cost of these treatments.
To reduce the complexity of the DCE, attributes were divided into 2 groups with even distribution regarding efficacy, safety and utilization features (group 1: ORR, PFS, type of AE, frequency of administration, frequency of consultations; group 2: 2-year survival rate, time to response, probability of AE-related treatment discontinuation, route of administration, frequency of consultations). The attribute “frequency of consultations” was included in both groups to allow later conversion. Examples of discrete choice scenarios are shown in Table SII.
Since combination of attribute levels resulted in 1,024 (4×4×4×4×4) possible treatment scenarios per attribute group, a fractional factorial design was applied. Twelve pairs of scenarios were assigned randomly to each participant for each attribute group, using an orthogonal design with balanced level overlap. Participants were asked to choose their preferred option for each combination of scenarios. Two fixed-choice sets, with one option clearly superior, were added as control questions to test for internal validity. Respondents who failed these control questions were excluded from statistical analyses, because they presumably did not understand the DCE. A pilot study with 10 participants was conducted to verify the relevance of the selected attributes and the comprehensibility of the task.
Part-worth utilities (PWU) and relative importance scores (RIS) were computed using Sawtooth Software. PWU for all attribute levels were determined using hierarchical Bayesian estimation, with positive values demonstrating high utility and negative values indicating disutility. RIS for each attribute were calculated by dividing the attributes’ utility range (highest to lowest) by the sum of the utility ranges of all attributes. To enable comparison between attribute groups, RIS were converted into a single list by matching the RIS of frequency of consultation for each participant. RIS were calculated individually for each attribute and each participant, and subsequently averaged across the study population.
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics version 25. If necessary, variables were transformed to approach normal distribution, e.g. with logarithmic transformation. For subgroup analyses, participants were stratified according to age (in years), sex (male vs. female), marital status (single or widowed vs. married or in a partnership), education (higher education entrance qualification (“Abitur” or “Fachabitur”, i.e. A-levels or vocational diploma), vs. low or intermediate school degree), AJCC 2017 stage (IIC vs. III vs. IV), present tumour burden (yes vs. no), and treatment experience with immune checkpoint inhibitors (yes vs. no), BRAF- and/or MEK-inhibitors (yes vs. no) or interferon-α (yes vs. no). Other systemic treatments, such as chemotherapy or other targeted therapies, were not considered for subgroup analyses due to the small numbers of cases (for details, see Table I). Differences in RIS and PWU between groups were assessed with analysis of variance (ANOVA) for categorical variables and Pearson’s correlation (PC) for continuous variables. Significance was set at p < 0.05. Differences between PWU within a single attribute were computed using within-group t-tests and significance was assumed for p < 0.001.
Associations between patients’ characteristics and preferences were investigated further using multivariate regression analysis. Nine models were created with RIS of each attribute as dependent variable and age, sex, marital status, education, tumour stage according to AJCC 2017, present tumour burden, treatment experience with immune checkpoint inhibitors, and treatment experience with BRAF- and/or MEK-inhibitors as independent variables. Employment status was not included due to multicollinearity with age. Standardized regression coefficients β, describing the change in RIS when the particular variable is modified while the others remain constant, were calculated for each independent variable. Again, p < 0.05 was considered significant.

Table I. Sociodemographic and disease-related characteristics of the study cohort
Study population
A total of 193 patients were invited to participate in the study. Of these, 174 agreed and provided written informed consent, and 162 completed the survey. Twelve participants were excluded due to incorrect answers to the control questions. These subjects did not differ significantly from the rest of the study cohort with respect to age, sex, marital status, education, school degree, time since diagnosis, tumour burden, treatment experience and current melanoma treatment (data not shown), but had a significantly lower tumour stage (p = 0.001 in χ2 test).
Data from 150 respondents who had passed the control questions were used for further analyses. The mean age of these participants was 58.6 years (range 23–85 years), 40% were female. 4.7% were in AJCC 2017 stage IIC, 58% in stage III, and 37.3% in stage IV. Melanoma manifestations were present in none of the patients in stage IIC, 16 of the patients in stage III (regional lymph node, satellite or in-transit metastases), and 42 patients in stage IV (all types of distant metastases). The mean time since initial diagnosis was 49.8 months. 61.3% currently received adjuvant or palliative systemic melanoma treatment. Of those, none were in stage IIC, 47.8% in stage III, and 52.2% in stage IV. Further characteristics are shown in Table I.
Preferences for treatment attributes
Averaged across all participants, the attributes regarded as most important were ORR (RIS 26.8) and 2-year survival rate (RIS 21.6), followed by type of AE (RIS 11.7) and probability of AE-related treatment discontinuation (RIS 9.2, Fig. 1A). PWU for immune-related AE (PWU –54.4) were significantly lower than utilities for AE typically associated with BRAF- and MEK-inhibitors or T-VEC (rash, photosensitivity reaction and warty hyperkeratosis: PWU 16.8; pyrexia, chills and flu-like symptoms: PWU 15.9; diarrhoea, nausea and decreased appetite: PWU 21.7; p < 0.001 in all pairwise comparisons).
Process attributes were considered less relevant. Among these, route of administration (RIS 8.0) was of greatest interest, followed by frequency of administration (RIS 5.0) and frequency of consultations (RIS 4.3). The preferred route of administration was intravenous infusion (PWU 16.1, p < 0.001 compared with intake of 6–12 tablets per day and intralesional injections), followed by 4–6 tablets daily (PWU 12.5, p < 0.001 compared with 6–12 tablets and intralesional injections), whereas intake of 6–12 tablets per day (PWU –9.7) and intralesional injections (PWU –18.9) had lower utilities.
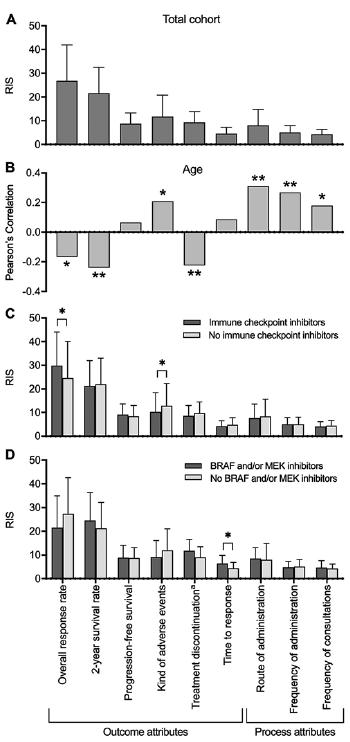
Fig. 1. Mean patient preferences for treatment attributes, influence of age and treatment experience. (A) Preferences averaged across the study sample. The attributes considered most important were overall response rate and 2-year survival, followed by the type of adverse events, whereas process attributes appeared less relevant. (B) Correlation between age and preferences. With increasing age, process attributes and the type of adverse events gained relevance, whereas efficacy was ranked lower. (C, D) Subgroup analysis according to experience with (C) immune checkpoint inhibitors and (D) BRAF- and/or MEK-inhibitors. Patients who ever received immune checkpoint inhibitors placed higher value on the overall response rate and cared less about the type of adverse events. Those experienced with BRAF- and/or MEK-inhibitors were more interested in time to response than others. aTreatment discontinuation due to adverse events. RIS: relative importance scores. Bars: means with standard deviation. *p < 0.05, **p < 0.01.
Impact of sociodemographic characteristics
With increasing age, respondents placed higher value on the type of AE (PC 0.209, p = 0.01) as well as on all process attributes (frequency of administration: PC 0.268, p = 0.001; mode of administration: PC 0.311, p = 0.0001; frequency of consultations: PC 0.179, p = 0.028). By contrast, ORR (PC –0.167, p = 0.041), 2-year-survival (PC –0.240, p = 0.003) and the probability of AE-related treatment discontinuation (PC –0.225, p = 0.006) were rated less relevant (Fig. 1B). These correlations were confirmed by multivariate regression analysis (Table II). Participants living in a partnership put higher emphasis on PFS than did single people (RIS 9.1 vs. 7.7, p = 0.025; Fig. S1A; β = 0.210, p = 0.014; Table II). Respondents with a higher education entrance qualification attached greater importance to 2-year-survival than did those with a lower level of education (RIS 23.6 vs. 20.0, p = 0.035), whereas the type of AE (RIS 10.1 vs. 13.2, p = 0.031), time to response (RIS 3.9 vs. 5.2, p = 0.015), route of administration (RIS 6.2 vs. 9.7, p = 0.003) and frequency of consultations (RIS 3.79 vs. 4.8, p = 0.007) were less essential from their perspective (Fig. S1B). These correlations were corroborated by multivariate regression analysis (Table II). Employed participants were significantly more interested in ORR (RIS 29.5 vs. 24.6, p = 0.037) and less concerned about the type of AE (RIS 10.4 vs. 12.9, p = 0.023), route of administration (RIS 6.4 vs. 9.5, p = 0.004) and frequency of administration (RIS 4.2 vs. 5.7, p = 0.001) than were unemployed or retired respondents (Fig. S1C). However, employed individuals were significantly younger than others (mean age: 48.4 vs. 67.3 years, p < 0.001) and 93% of respondents without employment were retired. There was no significant association between respondents’ sex and their preferences.
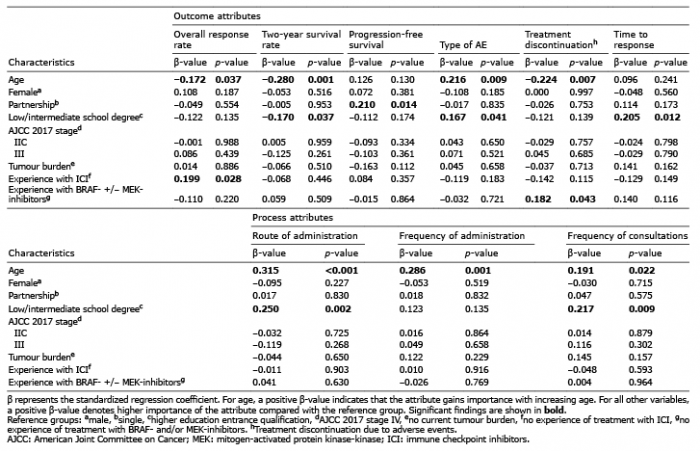
Table II. Multivariate regression models for outcome and process attributes
Impact of disease-related characteristics and treatment experience
Patients who had ever been treated with immune checkpoint inhibitors regarded ORR as more relevant than did others (RIS 29.8 vs. 24.6, p = 0.023; β = 0.199, p = 0.028; Fig. 1C, Table II) and worried less about the type of AE (RIS 10.2 vs. 12.9, p = 0.044). In particular, they were less concerned about immune-related AE (PUW –40.0 vs –65.4, p = 0.01) and stated lower utilities for AE-related pyrexia, chills and flu-like symptoms (PUW 8.4 vs. 21.7, p = 0.009). Subjects who had experienced treatment with BRAF- and/or MEK-inhibitors considered time to response more important than did others (RIS 6.5 vs. 4.4, p = 0.017; Fig. 1D). This finding could not be confirmed in multivariate regression analysis, possibly due to the small number of patients in the subgroup with BRAF- and/or MEK-inhibitors (n = 13). However, multivariate models suggested that participants who had ever been treated with BRAF- and/or MEK-inhibitors worried more about AE-related treatment discontinuation than did others (β = 0.182, p = 0.043, Table II). Prior treatment with interferon-α did not significantly influence preferences. Tumour stage, current tumour burden, time since diagnosis, and prior radiation therapy also did not correlate with patient preferences. Furthermore, experience of AE during the current or most recent systemic melanoma treatment did not impact preferences (data not shown).
The participants in this DCE study of patient preferences regarding treatments for melanoma considered efficacy most important, followed by safety, whereas process attributes were judged less relevant. Great focus on efficacy was also reported in other studies of patient preferences in treatment of melanoma (30, 31, 33–36) and other types of cancer (28).
DCEs investigating preferences for attributes of immune checkpoint inhibitors and targeted therapies were published recently by 3 groups (33–36). Stenehjem and colleagues performed a DCE in patients with melanoma and their cancer care providers in the USA (35). In accordance with the current results, they found that patients were particularly interested in overall survival (OS), immune-related AE, and skin toxicities, and less concerned about the route of administration, although their patient cohort was somewhat different from those in the current study. Seventy-five percent of their patients were in stage I or II, whereas the current study included only patients with high-risk melanoma (stage IIC–IV). The current study did not find a correlation between tumour stage or tumour burden and preferences, indicating that patients have high interest in treatment efficacy independent of these factors.
Liu et al. conducted a DCE in the USA comparing the preferences of patients with advanced melanoma with those of oncologists (33) or oncology nurses (34). Their discrete choice scenarios contained similar attributes to the current study, i.e. objective response rate, PFS, OS, grade 3/4 AE, mode of administration, dosing schedule and median duration of therapy. Attributes estimated as most important by patients were OS, followed by probability of grade 3/4 AE and objective response rate, whereas process attributes were considered less relevant, which was concordant with our findings and implies that the preferences of patients with advanced melanoma from Germany and the USA are similar, despite some differences in healthcare systems and cultural background. In contrast to the current results, Liu et al. did not observe a significant impact of age on patient preferences. However, the mean age of their study population (46.5 years) was lower than in the current study (58.6 years).
Mansfield and colleagues carried out a DCE and best-worst scaling exercise among individuals with a self-reported diagnosis of melanoma stage III or IV (36). Their discrete choice scenarios were composed of 4 attributes describing the risks of different AE, one efficacy attribute (PFS) and one process attribute (mode and frequency of administration). In line with the data from the current study, their respondents were willing to accept a higher risk of AE for improved survival. However, their subgroup analyses suggested that older participants placed higher value on PFS, whereas younger ones were more concerned about AE, in contrast to the current results.
Regarding the type of AE, patients surveyed by Stenehjem et al. feared immune-related AE more than skin toxicity and gastrointestinal AE (35). Participants in the study by Mansfield et al. were more interested in avoiding colitis, hormone gland problems and severe fever than in avoiding extreme sun sensitivity (36). Concordantly, the current study demonstrated the lowest PWU for immune-related AE.
In contrast to the DCE discussed above, the current study was conducted independently of the pharmaceutical industry. Moreover, the current study performed more comprehensive subgroup analyses with regard to sociodemographic, tumour-related and treatment characteristics, which were confirmed by medical record review, than the studies performed with support of the pharmaceutical companies.
Impact of sociodemographic characteristics
According to the current study, younger individuals placed greater value on ORR and 2-year survival than did older ones, which seems plausible. Younger patients were willing to trade AE and less convenient treatment schedules for a better chance of survival, and they were particularly afraid of treatment discontinuation due to AE. By contrast, older participants were more concerned about the type of AE and the treatment process than were younger ones, suggesting that they placed higher emphasis on the immediate effect of the therapy on their daily routine. This is understandable, as with increasing age the prevalence of morbidities also increases (37) and any reduction in quality of life represents an additional burden. Older patients with comorbidities have a higher risk of experiencing severe AE and a lower chance of recovering rapidly and fully from them than do younger, otherwise healthy, individuals. Regarding the treatment process, frequent consultations and having to take a large number of tablets may constitute a higher strain for elderly patients.
Participants with a higher education entrance qualification assigned greater importance to 2-year survival and lower value to the type of AE, time to response, route of administration and frequency of consultations than those with a lower school degree, implying that they are willing to endure AE and a less convenient treatment schedule for improved chances of survival. A lower education level was previously shown to correlate with greater tumour thickness of primary melanomas and increased mortality due to melanoma (38), possibly because patients with a lower education level are less aware of the clinical signs of melanoma and undergo less screening (39). This could also explain why our respondents with a lower level of education were less concerned about survival, but more interested in attributes that immediately affect everyday life.
Participants who were in a partnership valued PFS significantly more highly than did single participants. This is understandable, as they may feel that they have a responsibility to be available and take care of their partners or families.
Impact of treatment experience
Participants who had experienced treatment with immune checkpoint inhibitors were less concerned about the type of AE than were others. In particular, they were less afraid of immune-related AE, which were most feared in the whole cohort. It can be speculated that patients worrying less about immune-related AE were more likely to choose immune checkpoint inhibitors if faced with the choice between these medications and targeted therapies. Patients receiving immune checkpoint inhibitors were thoroughly informed about immune-related AE and their management before initiation of therapy. Many of them, already treated, may have found out that their immune-related AE were mild and/or manageable. Patients receiving a combination of nivolumab and ipilimumab were more likely to have preferences for efficacy and a particularly high willingness to accept high-grade toxicity. However, the number of patients treated with this combination was too small for further subgroup analysis.
A relatively high proportion of the study cohort had experience of treatment with interferon-a, which was the standard adjuvant treatment for melanoma in stage II and III in Germany until 2018. Remarkably, subgroup analyses did not reveal any significant effect of this experience on preferences, even though adjuvant treatment with interferon-α provides only limited benefit with respect to progression-free and overall survival at the price of potentially impairing AE, such as flu-like symptoms, fatigue and depression.
Patients with previous exposure to BRAF- and MEK-inhibitors were especially interested in a fast response, but it is possible that they may have been a selected group with high burden of disease; hence, fast response was crucial for them. Indeed, onset of action of BRAF- and MEK-inhibitors is faster than that of other treatments (40). Therefore, this approach is often chosen for patients with BRAF-mutated melanoma, high tumour burden and symptomatic metastases. The results of the current study suggest that patients receiving BRAF- and MEK-inhibitors value their rapidity of action; however, this may be influenced by how the clinician describes the drug to the patients, since otherwise they would not be aware of how quickly the drug was likely to act. On the other hand, these patients were particularly concerned about treatment discontinuation due to AE. The probability of high-grade toxicity leading to treatment discontinuation is comparable for BRAF- and MEK-inhibitor combinations and PD-1 inhibitor monotherapy, but significantly higher for nivolumab plus ipilimumab (10), an aspect that may be crucial when deciding in favour of a BRAF- and MEK-inhibitor combination and against nivolumab plus ipilimumab.
Limitations
A general limitation of DCEs is the hypothetical nature of the method. Participants are asked to make decisions that do not have real-world consequences. They may make different decisions if the consequences are real, which creates a limitation termed hypothetical bias. Furthermore, it is not possible to include all attributes that may be relevant for a treatment decision in a single DCE. For example, some attributes related to AE had to be neglected in order to limit the complexity of the choice tasks, which may result in omitted variable bias. When creating the DCE, it was decided not to include an opt-out-alternative in order to avoid loss in statistical efficacy, even though in reality refusing treatment is an option.
The number of participants was limited to 150. Of those, 56 were diagnosed with stage IV melanoma. Differences between subgroups, e.g. regarding tumour stage, might have been missed because of the limited cohort size. Moreover, the sample of patients who had experienced treatment with BRAF- and MEK-inhibitors was small (n = 13). Participants were recruited in clinics specialized in dermato-oncology in urban clinics. Preferences of patients living in rural region smay differ from those of patients living in urban areas due to more difficult access to healthcare facilities and longer journeys for consultations. To produce more generalizable data, the results of the current study require verification in a larger and more heterogeneous sample and in further dermato-oncology centres.
At the time of data collection, immune checkpoint inhibitors were approved for intravenous infusions every 2 or 3 weeks. Currently, infusions are also possible every 4 or 6 weeks, which may be more attractive for patients. The overall landscape of treatments for advanced melanoma is changing rapidly, and the introduction of novel medications or approval of new dosing regimens may impact on patient preferences.
Conclusion
This is the first DCE study on patient preferences regarding modern systemic therapies for melanoma that has been performed independently of the pharmaceutical industry. Averaged across the study sample, the results show that patients’ preferences are highest for efficacy, followed by safety. Preferences varied considerably depending on age, education and marital status, as well as on treatment experience. Patient-physician communication should address and explore patients’ individual characteristics, requirements, preferences and fears. Involving the patient actively in treatment decisions and acknowledging her/his individual preferences is essential to enhance treatment satisfaction, strengthen adherence and, ultimately, improve outcome.
Conflicts of interest: FK was a member of advisory boards for Amgen, Bristol-Myers Squibb (BMS), Roche, Novartis, MSD and Sanofi. BS participated in clinical trials for Array Biopharma, Janssen-Cilag, MSD and Sandoz, and received financial support from Janssen-Cilag for participation in conferences. JO served as investigator for Novartis and received financial support from Novartis for participation in conferences. VM conducted clinical trials for Amgen, Array BioPharma, BioNTech RNA Pharmaceuticals, BMS, MSD, Novartis, Roche and Sanofi and received financial support from Abbvie, Janssen-Cilag, MSD and Novartis for participation in conferences. JU is on the advisory board or has received honoraria and travel support from Amgen, BMS, GSK, LEO Pharma, MSD, Novartis, Pierre Fabre and Roche outside the submitted work. UH served as investigator for Magnosco and Novartis, was a member of advisory boards for Novartis and Takeda, received honoraria from AbbVie, Novartis and OmniaMed, and received support from Almirall Hermal, Amgen, Biofrontera, BMS, L’Oreal, MSD, Novartis, Roche, Pierre Fabre and Takeda for conferences. WH was a member of advisory boards for Novartis, received honoraria from Novartis and LEO Pharma, and received support from Abbvie, Almirall Hermal, Beiersdorf, Dermo Medical, Biofrontera, Celegene, Dermapharm, Dr. Pfleger, Galderma, Hexal, Janssen-Cilag, Jenapharm, Kosmetik vom Waßerfall, LEO Pharma, Medac, Novartis and Pfizer for conferences. WKP served as investigator for AbbVie, Array Biopharma, Boehringer Ingelheim, Eli Lilly, Janssen-Cilag, MSD, Novartis, Pfizer and UCB Pharma, was a member of advisory boards for Eli Lilly, LEO Pharma, MSD, Novartis, Pfizer, Roche and UCB Pharma, received honoraria from ALK-Abello, AbbVie, Biotest, BMS, Janssen-Cilag, MSD, Novartis, Pfizer, Dr. Pfleger and Roche, and received support from AbbVie, Actelion, ALK-Abello, Alma Lasers, Almirall Hermal, ARC Lasers, Asclepion, Beiersdorf, BMS, Celgene, Dermapharm, Dermasence, Eli Lilly, Galderma, GSK, Immunocore, Janssen-Cilag, L’Oreal, La Roche Posay, LEO Pharma, Medac, MSD, Mylan, Novartis, Pierre Fabre, P&M Cosmetics, Pfizer, Roche, Sanofi and Sun Pharma for conferences. JW, KD, M.-LS, MP and AK have no conflicts of interest to declare.
The study was performed without any support from the pharmaceutical industry. The conflicts of interest have no impact on the content of the manuscript.