Dermoscopic and Immunohistochemical Observations in Anetodermic Pilomatricoma: A Case Report
Department of Dermatology, Affiliated Hospital of Guangdong Medical University, 57 Southern Renmin Avenue, Xiashan District, Zhanjiang, Guangdong, 524001, China. *E-mail: ymfan1963@163.com
#These authors contributed equally to this work.
Accepted Feb 25, 2020; Epub ahead of print Mar 12, 2020
Acta Derm Venereol 2020; 100: adv00088
Pilomatricoma, or calcifying epithelioma of Malherbe, is a benign cutaneous neoplasm originating from hair matrix cells. Anetodermic, pseudobullous, or lymph-angiectatic pilomatricoma is a rare variant, and, to-date, approximately 30 cases have been reported (1–5). Although dermoscopy is helpful to diagnose non-anetodermic pilomatricoma (6–9), its application has been documented in only one case of anetodermic pilomatricoma (1). We describe here the clinicopathological, dermoscopic and immunohistochemical features of anetodermic pilomatricoma in a teenage patient.
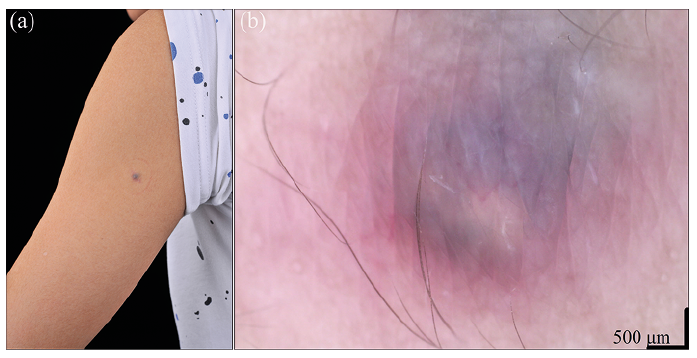
A 14-year-old healthy boy presented with a 7-year history of a slow-growing asymptomatic bluish nodule on his left arm. There was no history of previous local trauma or injection. Physical examination revealed a 5-mm elevated nodule with a bluish-reddish soft-wrinkled surface (Fig. 1a). Dermoscopy showed a bluish-greyish and yellowish-white central area with blurred linear-irregular vessels and reddish border (Fig. 1b). The tumour was completely excised and showed no relapse after 6 months.
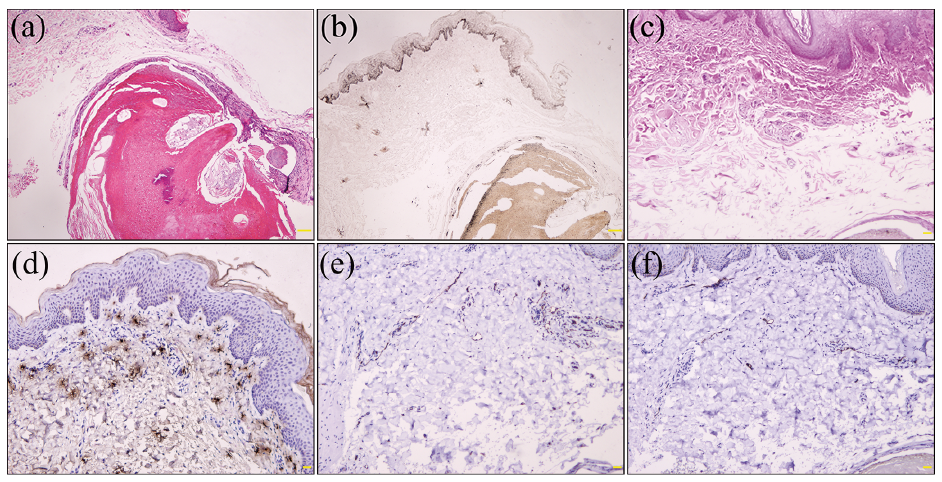
Postoperative histopathology showed mildly acanthotic epidermis and a well-demarcated dermal tumour composed of peripheral basaloid cells, central eosinophilic shadow cells and transitional cells. Focal calcification was present in the area of shadow cells (Fig. 2a), which was validated by alizarin red stain. Vascular proliferation and dilation and a few multinucleated giant cells were seen in the dermis. Fontana–Masson stain displayed hyperpigmentation in the basal layer of epidermis and basaloid areas of tumour (Fig. 2b). Elastic van Gieson stain showed the absence of elastic fibres in the supra-tumoural dermis (Fig. 2c). Immunohistochemically, tryptase stain showed incremental mastocytes in the dermis (Fig. 2d). CD31 stain revealed vascular and lymphatic proliferation (Fig. 2e), while D2–40 stain revealed lymphatic dilation (Fig. 2f). S-100 and Melan-A immunostaining revealed no melanocytes within the tumour. These findings were in accordance with a diagnosis of anetodermic pilomatricoma.
Anetodermic pilomatricoma generally manifests as a firm subcutaneous nodule with atrophic, wrinkled, keloid-like or pseudobullous brownish skin, and affects the upper arm, shoulder and trunk in young females (2, 3). Histopathologically, pilomatricoma is located in the dermis or subcutis and is composed of basaloid cells and ghost cells with focal calcification (4). Lymphatic dilation, disruption of collagen fibres, and absence of elastic fibres in the supra-tumoural dermis are the additional features of anetodermic pilomatricoma (2, 4). In line with 3 reported cases (3), mild acanthosis and basal pigmentation in the overlying epidermis and increased mast cells in the supra-tumoural dermis were also found in our case. In addition, the presence of focal pigmentation within and around basaloid nests seems to accord with pigmented pilomatricoma despite the lack of melanocytes in basaloid nests (10). Mast cells may contribute to epidermal proliferation, hyperpigmentation and elastolysis (3), while matrix metalloproteinase-9 and -12 derived from fibroblasts and infiltrating inflammatory cells can degrade elastic and collagen fibres (5), resulting in the brownish anetodermic appearance.
Preoperative diagnosis of pilomatricoma is accurate in 16% of 1,521 lesions (4). Its differential diagnoses include epidermal cyst, dermatofibroma, Spitz naevus, dermal melanocytic naevus, basal cell carcinoma (BCC) and melanoma (1, 2, 6). Dermoscopic features of nonanetodermic pilomatricoma include yellowish-white structures and streaks, reddish homogeneous areas, and polymorphous (hairpin, linear irregular, and dotted) vessels with ulceration and blue-grey structureless areas (6–9), of which irregular white structures/streaks and polymorphous/atypical vessels are more common (6). Comparably, red homogeneous areas, irregular white-yellow structures, irregular linear vessels, and blue-white veil were apparent in one case of anetodermic pilomatricoma (1), whereas bluish-greyish and yellowish-white central area with blurred linear-irregular vessels and reddish border were evident in our case. Yellowish-white structures and streaks correspond histopathologically to calcification or eosinophilic cornified masses in tumour centre, reddish homogeneous areas and polymorphous vessels represent vascular proliferation and dilation and local haemorrhages, and blue-grey structureless areas could be related to pigmentation within islands of basaloid cells, siderophages or melanophages in the dermis (1, 6).
Dermoscopically, an epidermal cyst on the face displayed irregular white structures and brown-blue central pigmentation with an erythematous border (11), but it can be excluded the diagnosis of pilomatricoma owing to the absence of polymorphous or atypical vessels. Although lateral compression often yields dimple-sign in dermatofibroma (12), vertical pressure also leads to central depression (2). The irregular whitish structures of pilomatricoma resemble the white scar-like patch of dermatofibroma, but a peripheral delicate pigment network is absent in pilomatricoma (6). Blue-grey structureless areas of pilomatricoma is similar to large blue-grey ovoid nests of BCC, but presence of irregular whitish structures and lack of other specific criteria (arborizing vessels, blue-grey globules, leaf-like and spoke wheel areas) of BCC support the diagnosis of pilomatricoma (6). Furthermore, melanoma always exhibits other features of melanocytic lesions including atypical pigment network, blue-white veil, and atypical dots/globules (6), while Spitz naevus possesses 3 dermoscopic patterns: starburst, regularly distributed dotted vessels and globular pattern with reticular depigmentation (13).
In conclusion, dermoscopy is useful to improve diagnostic accuracy in anetodermic pilomatricoma. Mastocyte infiltration may be involved in epidermal proliferation, hyperpigmentation and elastolysis of this entity.