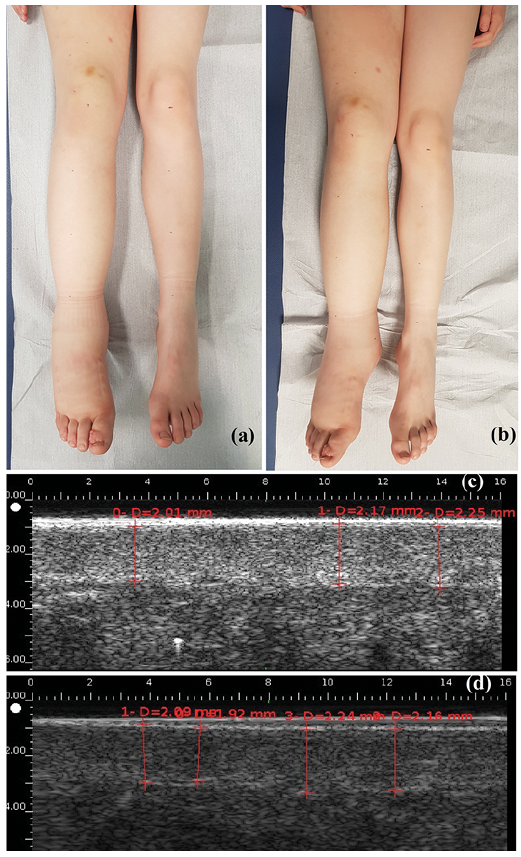
Fig. 1. Lymphoedema of the right lower limb in children (a) before and (b) after manual lymphatic drainage (MLD). High-resolution sonography measurements of dermal thickness (c) before and (d) after MLD.
1Department of Dermatology and Reference Center for Rare Diseases and Vascular Malformations (MAGEC) and 3Department of Physical Medicine and Rehabilitation Department, Centre Hospitalier Régional Universitaire de Tours, 2INSERM 1246 – SPHERE, Universities of Tours and Nantes, and 4INSERM U1253, University of Tours, Tours, France
#These authors contributed equally and should be considered as first authors.
Paediatric lymphoedema (LE) is a rare condition, for which there is little data available regarding treatments. The aim of this study was to assess the short-term effect and acceptability of a 30-min session of manual lymphatic drainage (MLD) in children with well-documented LE of the lower limbs. Fifteen children were included (8 males; median age 11 years). Comparison of the sum of circumference values for the whole limb before and after MLD revealed a slight, but significant, reduction (from a median of 289.8 to 285.5 cm, p = 0.024), but the limb volumes did not decrease significantly (from a median of 4,870.3 to 4,772.3 ml, p = 0.394). Dermal thickness, measured by high-resolution ultrasound, decreased from 1.44 to 1.40 mm (p < 0.001). All children reported improvement in well-being, and found MLD useful. In conclusion, MLD is well accepted by children, but has poor impact on LE swelling. However, it decreases cutaneous oedema by mobilizing the lymph fluid.
Key words: lymphoedema; children; ultrasound; lymphatic drainage; physiotherapy; cutaneous oedema.
Accepted Feb 25, 2020; Epub ahead of print Mar 11, 2020
Acta Derm Venereol 2020; 100: adv00125.
Corr: Annabel Maruani, Department of Dermatology, Centre Hospitalier Régional Universitaire de Tours, FR-37044 Tours Cedex 9, Tours, France. E-mail: annabel.maruani@univ-tours.fr
This study evaluated the immediate effect and acceptability of a single session of manual lymphatic drainage for paediatric limb lymphoedema. For each child the circumference of the affected limb was measured at many points, and the volume calculated. Skin thickness was measured with ultrasound. These measurements were made before and after the treatment. After the massage the children completed a questionnaire to evaluate the usefulness and acceptability of this treatment. Our results showed that this treatment had a slight, but significant, effect on skin thickness. Nevertheless, the limb volume reduction was not significant.
Lymphoedema (LE) is a rare condition in children; it affects approximately 1/100,000 people < 20 years old in occidental countries (1). LE is the clinical consequence of swelling within tissue caused by the accumulation of excess fluid and macromolecules in the interstitial space. Primary LE, the most frequent type in children, results from an abnormal constitutional lymphatic system, and secondary LE, from injured lymph nodes or lymphatic vessels, especially when lymph nodes are surgically removed. Breast cancer-related LE is the most prevalent LE in adults in western countries. Improvement in molecular biology techniques has led to a better understanding of primary LE, especially syndromic forms (2).
The natural history of LE is progressive worsening during life, with an increase in swelling, occurrence of cutaneous fibrosis on the LE limb, flares of cellulitis, functional impairment and decreased quality of life (3–6). The age of onset of swelling varies, and sometimes LE is detected during the prenatal period. Primary LE can be located on the lower or upper limbs, face or genitals. The diagnosis is based on physical examination, but complementary explorations, such as lymphoscintigraphy or MRI, might be performed for confirmation (7, 8).
There is no curative treatment for LE. Management of LE is a life-long process based on physical therapy and global preventive measures (3, 5, 9, 10). “Complete decongestive therapy” combines manual lymphatic drainage (MLD), skin care, exercise and compression bandaging. The aim of treatment is to limit progression of swelling, avoid cutaneous fibrosis, and prevent complications associated with LE (11, 12). The key aim of the treatment is improving quality of life. However, the optimal combination to maintain a long-term effect is uncertain. Moreover, the effectiveness of the individual components of such programmes have not been clearly shown to significantly reduce swelling, especially for MLD, for which studies of adults with LE showed contradictory results (13, 14).
No results of randomized controlled trials on the subject of LE in children have been published, and guidelines are extrapolated from adult LE, especially breast cancer-related LE. Compressive garments are used in children, although they are not always well accepted (3, 4, 9), and MLD techniques are widely used, because they seem acceptable and safe (13, 15). There are numerous MLD techniques, whose goals are similar (i.e. clear the regional lymph nodes of the limb toward normal adjacent lymph areas and thereby allow distal oedema of the limb to meet empty lymph reservoirs).
This study aimed to assess the short-term effect and acceptability of a single session of MLD performed by trained physiotherapists in children with limb LE.
A prospective study was conducted in a French tertiary care referral centre for LE and vascular anomalies (University Hospital Center of Tours, MAGEC). The study was performed in accordance with the principles of the Declaration of Helsinki. After information was provided to parents and children, parents provided their informed signed consent. The study was approved by the local ethics committee (#2018-102).
Participants
Inclusion criteria were age < 18 years, and well-documented primary or secondary LE of at least 1 limb, whatever the associated signs and syndromic forms. Exclusion criteria were: developmental delay and LE located exclusively on the face or genitals.
Protocol
Children were allowed following their usual treatment. Consultations took place in the morning for all patients. Demographic data and data on the LE history were collected, including current treatment and complications. For each consultation the LE limb was treated with MLD for approximately 30 min by a trained physiotherapist in our centre (PA, HT, or SB), who used a standardized MLD protocol derived from Földi’s technique (16) (the MLD protocol is shown in Appendix SI).
The circumference of the affected limb was measured with a tape-measure before and after MLD. Limbs were measured every 5 or 10 cm, according to the height of the child, at both sides of the elbow for upper limbs and the tip of patella for lower limbs. Limb volumes were calculated using the formula: V=(h/12π)×(C2+Cc+c2), where C is the largest circumference, c the smallest circumference, and h the distance between the largest and smallest circumference. For bilateral LE, the most swollen limb was included for measurements.
Dermal thickness was measured with high-resolution ultrasound (2020 Dermcup, Medica Atys, Soucieu en Jarrest, France), with a 25-MHz probe (17). The probe was held manually by the same operator (CEH), perpendicular to the surface, with minimal pressure. Three images of the dermis and hypodermis were taken at the site with the largest circumference, before and immediately after MLD (Fig. 1). The images were anonymized. Dermal thickness was measured on the images, and a mean was calculated by a dermatologist trained in high-resolution ultrasonography imaging (LM), who was blinded to the timing of the images regarding the MLD session.

Fig. 1. Lymphoedema of the right lower limb in children (a) before and (b) after manual lymphatic drainage (MLD). High-resolution sonography measurements of dermal thickness (c) before and (d) after MLD.
After MLD, children ≥ 6 years old answered a questionnaire regarding the MLD session. The questionnaire included 10 items reflecting subjective assessment of usefulness and acceptability of MLD. The questionnaire was developed by a panel of 4 physio-therapists and 3 medical doctors, who were familiar with managing LE in children and adults, on the basis of the Children-DLQI (18), the LyQLI (19) and the LYMQOL (20). It was then reviewed by 5 healthy children, aged 6–15 years, in order to determine whether the language was understandable. Questions about the child’s feelings before and during the MLD session (anxiety, pain, embarrassment, well-being) and self-reported usefulness of the MLD session, used a 4-point Likert scale ranging from 0, not at all, to 3, enormously).
Outcomes
Outcomes used to assess the short-term effect of MLD were the comparisons of data before and after MLD, as follows: (i) clinical measurements of limb circumference; (ii) calculations of limb volumes; (iii) dermal thickness, reflecting changes in cutaneous oedema, measured by high-resolution ultrasound. The outcome used for subjective assessment of usefulness and acceptability of MLD by children were answers to the questionnaire.
Statistical analysis
Continuous variables are described with medians and interquartile range (IQR) [Q1–Q3]. Categorical variables are summarized as number (%). Measures of the limb circumferences and calculated limb volumes were compared using non-parametric 2-sided Wilcoxon signed-rank test. Statistical analyses were performed with R v3.3.1. Two-sided p < 0.05 was considered statistically significant.
Characteristics of participants
Fifteen children with LE were included in the study between 1 November 2018 and 25 June 2019. LE was confirmed by a lymphoscintigraphy in 13 cases (86.7%). Eight children were males, and the median age was 11 years, IQR 7.5–13.0 (Table I). The LE was secondary to the surgical excision of an inguinal lymph node in one case (this allowed for excluding the diagnosis of lym-phoma, which had been suspected), and was primary in all other cases, with no identified genetic syndrome. LE was always located on the lower limbs and was bilateral in 7 (46.7%) cases.
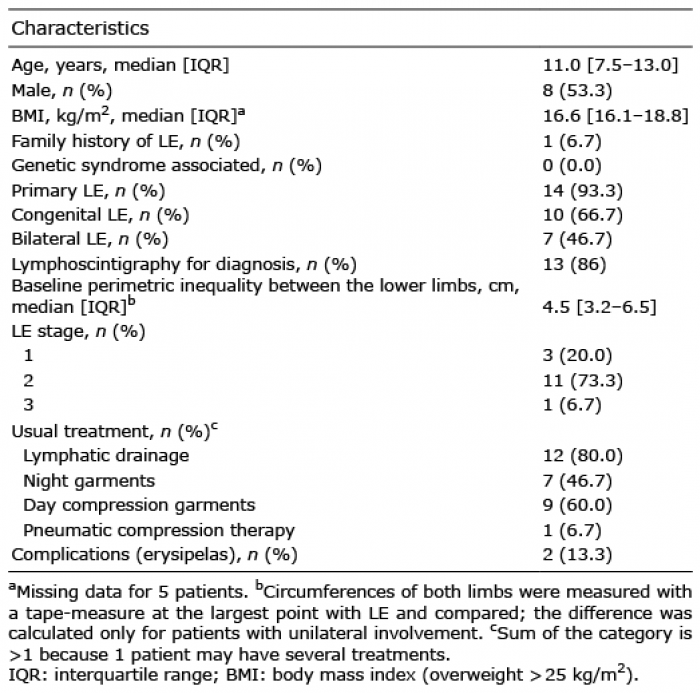
Table I. Characteristics of children with lymphoedema (LE) (n = 15)
Outcomes
Comparison of the sum of circumference values on the whole limb before and after MLD revealed a significant reduction, of 4.3 cm, in median value (from a median of 289.8 cm, IQR [203.2–317.2], to 285.5 cm, IQR [201.3–318.1], p = 0.024). Comparison of the limb volumes calculated before and after MLD revealed a non-significant reduction of 98 ml (from a median of 4,870.3 ml, IQR [1,827.1–7,371.5], to 4,772.3 ml, IQR [1,830.4–7,533.7], p = 0.390). Ultrasonographic dermal thickness on the largest limb circumference value decreased significantly after MLD, from a median of 1.44 mm, IQR [1.26–2.17] before MLD, to 1.40 mm, IQR [1.08–1.87] after (p < 0.001).
Twelve children were at least 6 years old and answered the questionnaire. All reported an improvement in well-being (from slight to major) after the MLD session and found MLD useful. Three reported experiencing little fear before the MLD session and little pain during MLD, and 5 felt a little embarrassed to undress and have a massage from the physiotherapist (Fig. 2).
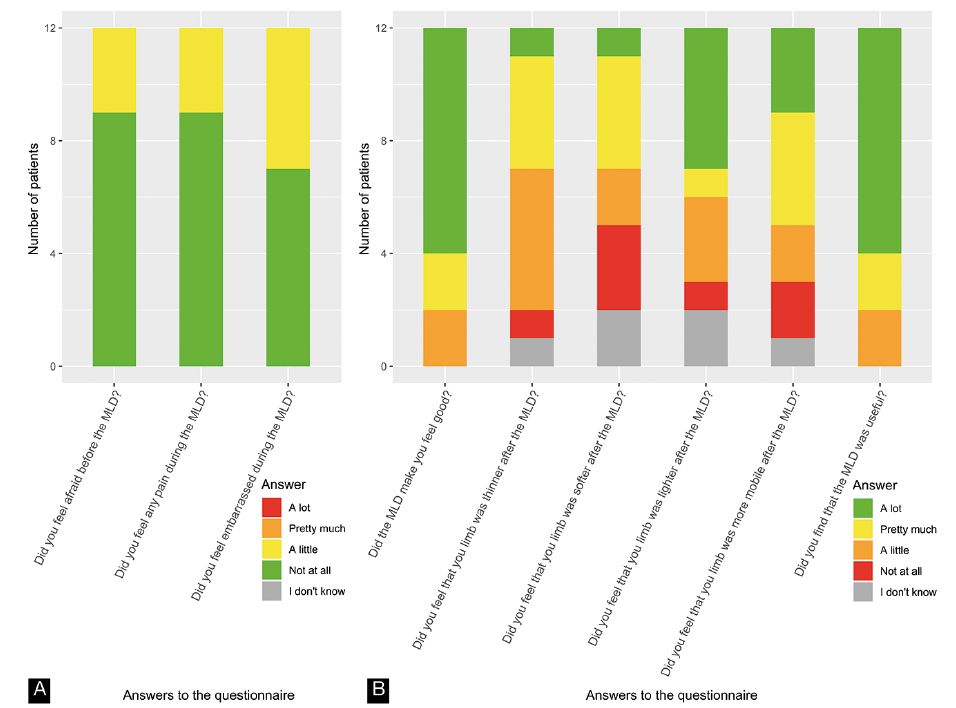
Fig. 2. Answers to 9 questions from the questionnaire regarding the efficacy and acceptability of manual lymphatic drainage (MLD).
For the 15 children with LE of the lower limbs who were included in this study, swelling of the limbs decreased only slightly after a single session of MLD. This decrease was statistically non-significant when considering the volume calculation, and was statistically significant, but not clinically relevant, when considering the sum of limb circumferences. However, ultrasound measurement showed a significant decrease in median dermal thickness, from 1.44 to 1.40 mm. All children reported improvement in well-being after MLD and found MLD useful; 5 felt a little embarrassed to undress and have a massage from the physiotherapist.
Interpretation
The very slight decrease in swelling of LE limbs in this study might be explained by 2 contradictory hypotheses. The first considers that MLD is immediately effective, but that this is not evidenced in our study, because the children already had optimal treatment. Most were already being treated with MLD and compression garments, which may have led to an underestimation of the immediate effect of MLD. Indeed, a decrease in skin thickness, which is a very sensitive measure reflecting a decrease in skin oedema, was evidenced here. Moreover, MLD has previously been shown to induce immediate modification of the flow of lymphatic fluid, by using lymphoscintigraphy, in a cohort of 16 adults with LE (21). In addition, a study of 9 healthy volunteers who underwent near-infrared imaging showed that MLD increased lymphatic activity (22) by improving the displacement and speed of lymph fluid, stimulating lymphatic accessory routes and decreasing dermal reflux.
The second hypothesis favours the non-efficacy of MLD in reducing LE swelling. In the literature, the results show a positive impact of compressive therapy on LE (high- and low-stretch bandages) (23), but the results are controversial when considering MLD (13, 14). Two systemic reviews assessing the effect of MLD concluded that MLD was safe, but the additional benefit to compression therapy was not clearly evidenced and further studies were needed (13, 24). However, the trials assessed different methods of MLD, at different frequencies. No studies that tested the optimal number of MLD sessions were found, but adherence with MLD would probably be poor for children on the long term with too-frequent sessions. In addition, outcomes used in trials were highly heterogeneous. They mainly included self-reported assessments, volumetric change measurements, and measurement of dermal thickness with high-resolution ultrasound. The patient’s well-being should always be considered during treatment, especially for children, because compliance is a key point in maintaining long-term treatment in chronic diseases (25).
In this study, a clinical outcome was chosen based on measuring limb volume calculated from circumference measurements, even though this is not a very precise method, because it was easy to measure and reliable in clinical practice to evidence supra-centimetric changes (26). High-resolution ultrasound appears to be a good way to distinguish LE, with hypoechogenicity including the whole dermis, from other causes of swelling, such as lipoedema (27). Indeed, LE affects the dermal and subcutaneous layers of the skin by increasing its water content, and an increase in dermal water content directly influences dermal thickness.
LE negatively affects quality of life and body image (28, 29). MLD can have positive effects in terms of emotional function, sleep disturbance, and pain and heaviness in adults with breast cancer-related LE (30). However, no study has assessed these effects of MLD in children. In the current study, children reported the utility of the session and improvement in well-being. However, several subjects said they were a little anxious before the session, and more than a third (n=5/12) reported that they felt a little embarrassed. Practitioners should be careful not to over-intrude and should take into account the children’s modesty.
Study limitations
The main limitations of this study were: (i) the small number of patients; however, LE is very rare in children, and this is the first prospective study evaluating an intervention; (ii) only the immediate effects of MLD were evaluated in children whose LE was already treated with compression garments and MLD; (iii) possible human error in measuring limb circumference with a tape-measure.
Conclusion
This study shows that MLD is well accepted by children, but has poor impact on LE swelling. However, it decreases cutaneous oedema by mobilizing lymph fluid. This may prevent fluid accumulation and subsequent fibrosis of the skin. Studies of the long-term efficacy of MLD in these patients are needed.
The authors thank Mrs Emiliène Edée, Reference Center for Rare Diseases and Vascular Malformations, CHRU Tours, for technical help.