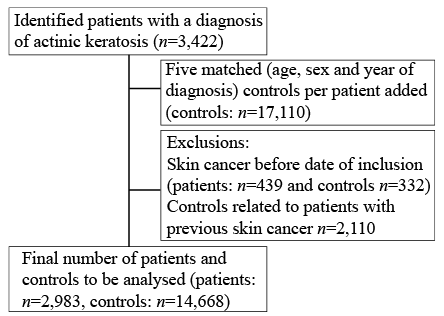
Fig. 1. Flow chart of the distribution of study participants, by means of patients with actinic keratosis and matched controls.
1Department of Health, Medicine and Caring Sciences, 2Department of Biomedical and Clinical Sciences, Division of Dermatology, Linköping University, and 3Research and Development Unit in Region Östergötland, Linköping, Sweden
Actinic keratosis is the most common actinic lesion in fair-skinned populations. It is accepted as an indicator of actinic skin damage and as an occasional precursor of squamous cell carcinoma. The aim of this study was to investigate, in a cohort of patients with a diagnosis of actinic keratosis, the relative risk of developing skin cancer during a follow-up period of 10 years. This registry-based cohort study compared a cohort of 2,893 individuals in south-eastern Sweden, who were diagnosed with actinic keratosis during the period 2000 to 2004, with a matched-control cohort of 14,668 individuals without actinic keratosis during the same inclusion period. The subjects were followed for 10 years to identify skin cancer development in both cohorts. Hazard ratios with 95% confidence intervals (95% CI) were used as risk measures. Individuals in the actinic keratosis cohort had a markedly higher risk for all skin cancer forms compared with the control cohort (hazard ratio (HR) 5.1, 95% CI 4.7–5.6). The relative risk was highest for developing squamous cell carcinoma (SCC) (HR 7.7, 95% CI 6.7–8.8) and somewhat lower for basal cell carcinoma (BCC) (HR 4.4, 95% CI 4.1–5.0) and malignant melanoma (MM) (HR 2.7 (2.1–3.6). Patients with a diagnosis of actinic keratosis were found to be at increased risk of developing SCC, BCC and MM in the 10 years following diagnosis of actinic keratosis. In conclusion, a diagnosis of actinic keratosis, even in the absence of documentation of other features of chronic sun exposure, is a marker of increased risk of skin cancer, which should be addressed with individually directed preventive advice.
Key words: skin cancer; actinic keratosis; cohort study; melanoma; squamous cell carcinoma; basal cell carcinoma.
Accepted Apr 21, 2020; Epub ahead of print Apr 21, 2020
Acta Derm Venereol 2020; 100: adv00128.
Corr: Magnus Falk, Department of Health, Medicine and Caring Sciences, Linköping University, SE-581 83, Linköping, Sweden. E-mail: magnus.falk@liu.se
Actinic keratosis is a common skin lesion associated with chronic exposure to sun. In most cases actinic keratosis is harmless, but it occasionally transforms into squamous cell carcinoma. This study included 2,893 patients with actinic keratosis, and investigated their 10-year risk of developing skin cancer, compared with a control group of 14,668 patients without actinic keratosis. Patients with actinic keratosis were found to have a more than 5 times increased risk of getting skin cancer. With regard to specific types of skin cancer, this increased risk in patients with actinic keratosis was highest (greater than 7 times higher) for squamous cell carcinoma, greater than 4 times higher for basal cell carcinoma, and almost 3 times higher for malignant melanoma. In conclusion, actinic keratosis is an important indicator of increased risk of skin cancer.
Actinic keratosis (AK) is a common skin condition caused by long-term exposure to the sun in susceptible individuals. AK typically develops on sun-exposed areas, such as the face, neck, balding scalp, chest, shoulders, and the back of arms and hands of mainly Caucasian adults (1–5), presenting as a rough, dry, scaly or crusted lesion which can be skin-coloured or tanned, sometimes with an erythematous base. Clinically, AK is sometimes difficult to differentiate from other benign skin conditions, such as lichenoid keratosis and other benign keratotic lesions (6, 7). AKs are often asymptomatic, but can sometimes be sore or itchy (1, 8). The diagnosis of AK is, in practice, often set clinically, based on its typical appearances, without histopathological confirmation, and the condition is therefore not regularly recorded in pathology databases (4, 8). Data about the prevalence of AK are thus relatively sparse, and originate mostly from Australia and the USA, with only a few studies available from Europe and Asia and no information from Africa and South America (9, 10).
On the one hand, as is the case for the majority of lesions, a single AK lesion may be completely harmless, with only cosmetic consequences for patients. On the other hand, however, the potential for malignant transformation is well documented; AKs are the most common precursor of invasive SCC (11). Any single AK lesion may have 1 of 3 possible outcomes: it can enter spontaneous remission; remain stable without further progression; or transform over time into in situ or invasive squamous cell carcinoma (SCC) (1, 2, 12). The reported risk of malignant progression of AK is widely variable. A systematic review found that the estimated risk of a single AK lesion becoming malignant ranged between 0.075% and 0.096% per year, or approximately1% over 10 years, with some estimates as high as 10% over 10 years (2). In another review the risk of progression of AK to invasive SCC varied between 0.025% and 16% per year (12). AK, and non-melanoma skin cancer (NMSC), are both also risk factors for the development of malignant melanoma (MM); a study in Italy (13) has shown that more than 40% of patients with a previous diagnosis of multiple AKs developed a NMSC or a MM during a follow-up period of 5–11 years. Solid-organ transplanted patients, such as those with heart, lung and kidney transplants, are at specifically increased risk of developing malignancies, including skin cancer and, especially, SCC, due to immunosuppression (14–19). The risk of SCC has been found to increase for those with more than 5 AKs, and the majority of SCCs arise from AKs (2). AKs on sun-exposed body surfaces indicate previous chronic exposure to ultraviolet (UV) radiation, and may, together with other factors, such as age, duration, and skin type, be sufficient to facilitate malignant change. Just as for basal cell carcinomas (BCC) and SCC, the prevalence of AK is typically higher in sun-intense geographical regions, but the specific pathogenic factors associated with progression of AK to SCC are not clear (20).
Although the most common reason for treatment of AK is prevention of malignancy, lesions are also treated for cosmetic purposes and to provide relief from symptoms, such as tenderness or itch (4), and can either be lesion directed, such as curettage and cryotherapy, or field directed, by means of a variety of topical, pharmacological treatments and photodynamic therapy (PDT) (1, 8, 21). Some national guidelines or consensus reports recommend treatment of AK, and subsequent clinical follow-up of treated patients, due to its malignant potential (22, 23), whereas other are less dogmatic (24). Routine obligatory treatment of AK would entail a substantial burden on general practitioners and on dermatological specialist care, which, in many cases, is already strained by the diagnostic process and care of increasing number of cutaneous malignancies (4).
With increasing incidence of skin cancer, research into prevention and early detection measures to counteract its development is important, not least from a primary healthcare perspective. This includes the identification of individuals who are at particular risk of developing skin cancer, among whom patients with AK emerge as an identifiable and potentially important category. The aim of the present study was to investigate, in a cohort of patients with a documented diagnosis of AK, the relative risk of developing skin cancer during a follow-up period of 10 years.
The study was approved by the Regional Ethics Review Board in Linköping, Sweden (number 2015/182-31).
The study was a registry-based cohort study on patients resident in the County of Östergötland, south-eastern Sweden, which has a total population of approximately 453,600 inhabitants (2017). As population-based data source, an administrative healthcare registry, The Care Data Warehouse in Östergötland, (CDWÖ) was used. The registry consists of administrative records of all publicly produced healthcare utilization in the county, including inpatient and outpatient care for all medical specialties, covering more than 95% of the total healthcare utilization in the county, from 1998 onwards (25). All patients aged 18 years or over who were clinically or histopathologically diagnosed with AK (ICD-10 code L57.0), during a 5-year time-period from January 2000 to December 2004, were identified from the registry as the AK cohort. The date of diagnosis of AK was set as the patient’s index date. Patients were included independently of the number of lesions or form of treatment, and were only included once, even if they were treated on more than one occasion. Patients were excluded if they had a previous history of skin cancer (in the form of MM (ICD-10 code C43.0–C43.9), SCC (ICD-10 code: C44.0S–C44.9S) or BCC (ICD-10 code: C44.0D–C44.9D)) prior to the study baseline, according to data from the national Swedish Cancer Register. The Swedish Cancer Register contains data on MM and SCC from January 1958 and on BCC from January 2004.
As a control cohort, up to 5 matched individuals without a diagnosis of AK during the inclusion period 2000 to 2004 were identified in the CDWÖ. As in the study cohort, individuals with a previous history of skin cancer were excluded based on data from the national Swedish Cancer Register. Remaining cases were included in the control cohort, frequency-matched by age, sex and index year. The corresponding index date was used to set the start date for the matched controls.
During a follow-up period from baseline to 2014, data from the national Swedish Cancer Register was used to identify whether the participants had been diagnosed with MM, SCC or BCC. Each individual was monitored from the index date until diagnosis with skin cancer, or until they were omitted due to loss to follow-up, death, or until the end of the study in 2014. Events of MM, SCC and BCC were identified for each cohort.
Statistical analysis
A χ2 test was used to analyse categorical data, and an independent t-test to analyse continuous data. The Kaplan–Meier method was used to estimate the cumulative incidence for patients and controls for different endpoints (1: all skin cancers; 2: SCC; 3: BCC; 4: MM), and multivariable Cox regression, with the same endpoints as listed above, to determine the hazard ratios (HR) and 95% confidence intervals (95% CI) between patients and controls. To investigate whether the HRs between patients and controls varied according to sex and age, the sex- and age-specific HRs were calculated for each endpoint and the p-values for the interaction with group were presented. All Cox models were adjusted for age and sex.
The median follow-up time was 10.6 years (range 0.1–14.99 years). Follow-up was stopped if the person had an event. Consequently, if there were fewer events in a group, then the follow-up time would be longer in that group. Patients in the AK cohort were thus followed for a shorter time compared with the control cohort 9.6 (0.1–14.99) and 10.7 (0.1–14.99) years, respectively.
Eligible study individuals consisted of 3,422 individuals in the AK cohort and 17,110 individuals in the non-AK control cohort. A history of prior skin cancer was an exclusion criterion. In the AK cohort 439 individuals (12.8%) were excluded, whereas only 332 (1%) individuals were excluded from the control cohort. After exclusion of individuals with a previous history of skin cancer the AK cohort comprised 2,983 patients and the control cohort comprised 16,778 individuals. If an AK patient was excluded due to previous skin cancer, its identified matched controls were also excluded, unless they matched another AK patient with fewer than 5 identified controls (85 of 2,110 excluded controls were re-matched to the AK cohort). In the analysable material, 2,983 individuals remained in the AK group and 14,668 in the control group (Fig. 1).

Fig. 1. Flow chart of the distribution of study participants, by means of patients with actinic keratosis and matched controls.
The characteristics of the study cohort of patients with AK and the matched controls are shown in Table I. There was a somewhat greater proportion of women (56.3%), and a higher representation of individuals within the age interval 70–79 years, representing 55.9% of our population. The lack of statistically significant differences between the 2 groups regarding sex, age and year of inclusion confirmed that the controls were successfully matched to the patients.
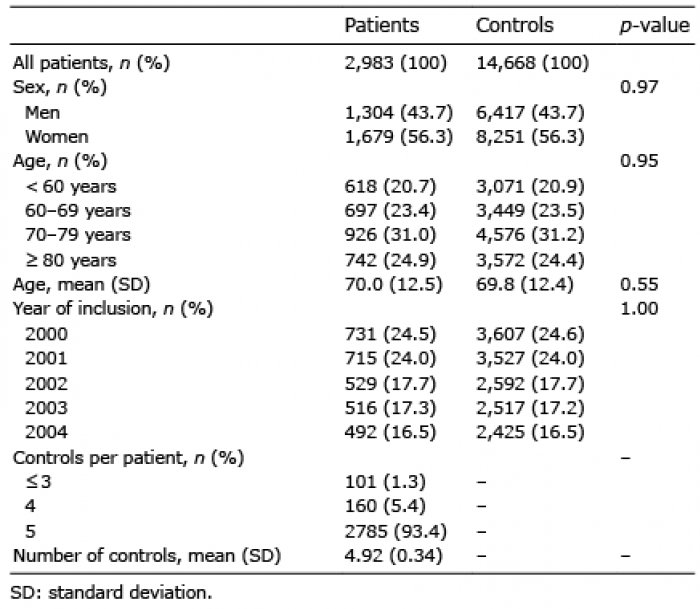
Table I. Characteristics of the patients in the actinic keratosis cohort and the control cohort
Table II shows the cumulative incidences of SCC, BCC and CMM in the 2 cohorts, and also the HR for each diagnosis. As seen, the AK cohort had a higher risk for all 3 cancer forms than did the control cohort. Patients with AK had 5.1 (95% CI: 4.7–5.6) times higher risk of developing some form of skin cancer within 10 years compared with the control group, i.e. individuals without AK. The difference was significant for all types of skin cancer, but most pronounced for SCC.
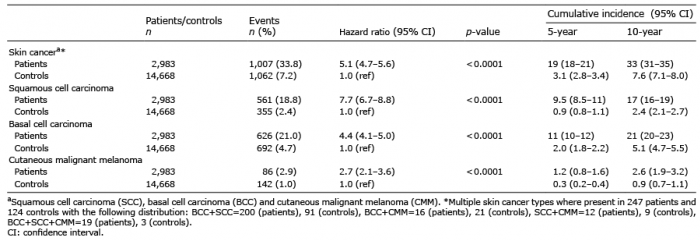
Table II. Hazard ratio and 5- and 10-year cumulative incidence of skin cancera in 2,983 patients with actinic keratoses (AK) compared with 14,668 matched (age, sex and year of diagnosis) controls
Table III shows the HRs for each of the different skin cancer types, as well as for all skin cancers. The HR of developing BCC was significantly lower for women than for men (pinteraction< 0.001). The age group ≤ 59 years had the highest HR of developing SCC (pinteraction< 0.01) and BCC (pinteraction< 0.01) compared with the other age groups.
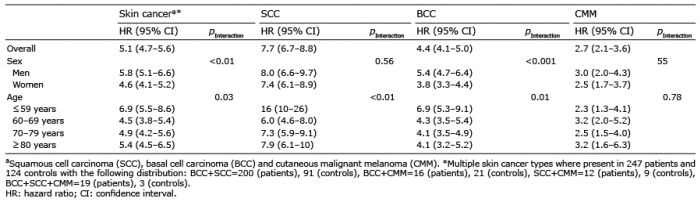
Table III. Hazard ratios, stratified by sex and age, for the incidence of skin cancera in 2,983 patients with actinic keratoses (AK) compared with 14,668 matched (age, sex and year of diagnosis) controls
The most important result of this study is the finding of a strong relationship between a diagnosis of AK and development of skin cancer. Patients with AK were found to have a 5 times higher risk of developing some form of skin cancer during 10 years’ follow-up compared with patients in the control cohort. This was more prominent for patients with AK aged ≤ 59 years at inclusion, for which the HRs for developing BCC and SCC were particularly high (6.9 and 16, respectively). Another notable finding was that the exclusion criteria “previous skin cancer” was applied for 12.8% of the AK group, but for only 1% of the control group, indicating the general association of AK with both keratinocyte and melanocyte malignancy.
Study limitations and strengths
This study has some limitations. Firstly, the diagnoses are based on registration in the local registry (CDWÖ), which, although it covers 95% of all cases reported in patients’ medical records (25), does not provide data on probable under-reporting of AK. Under-reporting is likely, not only because an unknown number of patients probably never seek medical help for their AK, but also because the diagnosis is, in most cases, set clinically, and not by histopathology, hence it is not certain that the diagnosis is always correct. In addition, an AK may be discovered as a secondary diagnosis, in which case the likelihood of its registration as a diagnosis can be expected to be lower than for a primary diagnosis. Finally, it is important to consider that the results of this study do not refer to patients with AK in general, but only to those who choose to seek healthcare.
Another limitation is that registration of BCC was not initiated before January 2004, i.e. after the start of our inclusion period; hence, cases of BCC may also have been under-reported in both cohorts. An additional limitation is that the ICD coding mixes all subtypes of SCC and MM. In cases of uncertainty during the diagnostic process, the physician registers the non-specific diagnosis code “other and non-specified skin changes” before having the histopathological answer. The actual frequency with which the histopathology report resulted in a revision of the initial diagnosis set at the time of clinical examination is unknown. Neither does the data collection allow for determination of whether there were multiple (or single) AKs or whether solar lentigines or actinic elastosis (common other features of “actinically damaged skin”) were present. Nor is it possible to draw any conclusions about possible connections between sites of AK, possible treatment modality and subsequent skin cancer development, which prohibits any interpretation of the possible malignification of an individual AK.
An important strength of the study is that it includes healthy individuals in the control group, i.e. not only those who had had contact with healthcare. This is an advantage, since these are, as a group, closer in character to a general population. Another strength is that this is a full population-based study, based on a whole county, thus eliminating selection biases. The 10-year follow-up interval may be discussed and may have been extended, but we think that it is a reasonably long time period for this age group and sample size of individuals to present with a sufficient incidence of skin malignancies, and to detect and compare differences with the control cohort. Overall, and despite the limitations mentioned above, we believe that this relatively large cohort study provides novel and important information concerning the risk of skin cancer development in patients with AK.
The HR for patients with AK to develop SCC was much higher (approximately double) than the corresponding HRs for MM and BCC, possibly because there is a stronger relationship between AK and SCC in addition to the general effect of chronic sun exposure. Regarding the proportion of patients with AK developing any form of skin cancer (SCC; BCC; MM), the current study showed this to be somewhat lower (33.8%) than in an Italian study by Dika et al. (13) (41.5%), even though we had a longer follow-up period. This may be due to possible discrepancies in documentation of skin cancer diagnosis in the different registries used for the studies, or other selection biases, but it cannot be excluded that the Italian population, for some reason, may have a higher risk of developing skin cancer. It is likely that the difference in insolation between the study populations lies behind this difference in outcome, although this requires further research. There was a female dominance in our AK cohort (56%, see Table I), a finding that is in contrast with that of previous studies (1–3). On the other hand, men had a higher risk of developing skin cancer compared with women (Table III). This is in concordance with other studies (1–3, 26). We also noticed that women had significantly lower HRs for developing BCC compared with men (3.8 vs. 5.4).
The localization of AK lesions and developed skin cancers were not specified in our study, since we wanted to assess the relative risk of developing skin cancer in general, unrelated to the body location of a specific lesion. Neither did we have access to information about the treatments received by the patients with AK and its possible effect on individual risk of developing skin cancer from the specific (or other co-existing) lesion treated. The considerable differences in risk levels for cancer development from a single AK (from 0.025% to 16% per year), shown in previous studies (12), is probably related to the fact that the studies were conducted in different modalities. Also, in our study the median follow-up time of patients with AK was shorter than that of controls, due to a larger number of events.
This study has some bearing on the logic of management issues in patients with AK. Whilst the current study has little to add to the question of the fate of an individual with AK, the results support the consensus of opinion that the likelihood of progression is extremely small. On the other hand, the fact that a clinical diagnosis of AK has been made is clearly associated with an increased risk for future (or present) skin cancer at an undetermined site in the same patient. Although not evaluated in our study, histopathological features of AK can, as described previously (27), be correlated with the risk of developing other cutaneous neoplasms. Thus, we regard AK as a marker of chronic sun exposure over the patient’s lifetime, increasing the risk of skin cancer, rather than AKs per se being dangerous. In conclusion, this calls for the provision of structured prevention information for patients even with their first AK, and of providing information on early detection. A consequence of that is the necessity for the healthcare systems to meet the patient’s need for repeated examination as required. The question of whether a planned follow-up should be provided requires further discussion, since healthcare resources are not unlimited and efforts may be best put into making the routines for “as required” examinations more effective. There is currently no existing technology or prognostic markers that allow clinicians to distinguish between individual lesions of AK that will resolve, remain stable, or progress to invasive disease, thus the treatment (or not) of an AK lesion is left to an individual, clinical management decision.
Conclusion
In conclusion, patients for whom a clinical diagnosis of AK has been set have a considerably higher risk of developing SCC, BCC or MM compared with sex- and age-matched controls, within 10 years from diagnosis. Although an individual AK is usually considered a harmless skin lesion, with a considerable rate of spontaneous resolution and with progression to SCC as a relatively rare event, the results of this study illustrate an opportunity to use the identification and documentation of AKs, even without documentation of other manifestations of solar damage, as motivation for the targeted provision of appropriate skin cancer prevention information. This may be an important and achievable part of our efforts to reduce the incidence and burden of skin cancer.
The authors have no conflicts of interest to declare.