There is a recognized need to better understand changes in the epidemiology of psoriasis and psoriatic arthritis (PsA) over time in Asia. Using the Taiwan National Health Insurance claim records this population-based study examined changes in the prevalence, incidence, and mortality rates in patients with psoriasis or psoriatic arthritis in Taiwan over 12 years. Patients with ≥1 diagnosis code for psoriasis or psoriatic arthritis, recorded either by dermatologists or rheumatologists, were identified. Annual age- and sex-standardized prevalence and incidence rates were calculated using the Taiwan general population as reference. To investigate mortality, each patient in the incident cohort was matched to 10 comparators from the general population by sex and age (at diagnosis). The risk of mortality between study cohorts and comparators was analysed by Cox proportional hazard regression. The prevalence of psoriasis (0.18–0.86%) and psoriatic arthritis (0.01–0.08%) increased steadily between 2006 and 2017. The incidence rates, however, remained stable (psoriasis: 62–65 per 100,000 person-years; psoriatic arthritis: 6–5 per 100,000 person-years). The risk of all-cause mortality for patients with psoriasis (hazard ratio 1.16; 95% confidence interval: 1.13–1.19) was higher than the general population, despite a decreasing trend over time in the all-cause mortality rates for both groups. The steady increase in the prevalence of psoriasis despite stable incidence rates suggests that improvements in life expectancy may be the key determinant of this increase.
Key words: psoriasis; psoriatic arthritis; epidemiology; incidence; prevalence; mortality.
Accepted Sep 6, 2022; Epub ahead of print Sep 6, 2022
Acta Derm Venereol 2022; 102: adv00807.
DOI: https://doi.org/10.2340/actadv.v102.1962
Corr: Ireny Y. K. Iskandar, Centre for Occupational and Environmental Health, Division of Population Health, Health Services Research and Primary Care, School of Health Sciences, The University of Manchester, Devas Street, Ellen Wilkinson Building, Manchester, M13 9PL, UK. E-mail: ireny.iskandar@manchester.ac.uk
SIGNIFICANCE
In 2014, the WHO recognized psoriasis as a serious non-communicable disease and its report emphasized the need to better understand the global burden of the disease. However, epidemiological information on psoriasis or psoriatic arthritis in Asia is limited. This population-based study found that, over 12 years, the incidence of psoriasis and psoriatic arthritis in Taiwan remained stable; however, the prevalence increased steadily over time. The steady increase in prevalence of psoriasis despite stable incidence rates suggests that improvements in life expectancy may be the key determinant of this increase.
INTRODUCTION
Psoriasis is a chronic, immune-mediated inflammatory skin condition (1), which is now considered a systemic disease associated with psychological, metabolic, arthritic, and cardiovascular comorbidities (2). Consequently, lifespan is reduced (3). In 2014, the World Health Organization (WHO) recognized psoriasis as a serious non-communicable disease (4) and the accompanying WHO report (2016) emphasized the need to better understand the global burden of the disease (4). In Taiwan, the National Health Insurance (NHI) provides a wide range of health services and treatments to patients with psoriasis and psoriatic arthritis (PsA) (5). However, with a rapidly ageing population and increasing medical expenditures in Taiwan, there is a recognized need to further understand the epidemiology of psoriasis and PsA.
To date, there are important knowledge gaps in understanding the natural history and disease burden of psoriasis and PsA in Asia (6, 7). Specifically, previous studies from Taiwan have provided limited information on the epidemiology of psoriasis and PsA based on age and sex, and on the temporal trends of the incidence and prevalence (8–11). A recent study has reported excess mortality in patients with psoriasis and PsA compared with the general population (12). Nevertheless, no studies have examined temporal trends in all-cause mortality in patients with psoriasis and PsA in Taiwan. It is critical to determine temporal trends as they will impact on disease prevalence. To date, no studies have simultaneously explored longitudinal trends in incidence, prevalence and mortality in patients with psoriasis or PsA in Taiwan.
Understanding the epidemiology of psoriasis and PsA in Taiwan is vital in order to quantify the social and economic burden of the diseases and to inform policy decisions on the delivery of healthcare services and resource allocation to reduce the morbidity associated with the conditions (4, 13). Therefore, the aim of this study was investigate the epidemiology of psoriasis and PsA in Taiwan. The specific objectives of the study were to determine trends in the incidence, prevalence and mortality of patients with psoriasis and PsA in Taiwan and examine how these epidemiological factors changed over time.
METHODS
Study design and data source
This population-based cohort study used the Taiwan NHI claim database and death certificates from 1 January 2006 to 31 December 2017. The publicly funded NHI programme (launched in 1995) provides a very comprehensive benefit package, including outpatient and inpatient services, prescription drugs, Chinese herbal remedies, and Western and preventive medicine to 99.9% of the 23.5 million people in Taiwan (5). The NHI research database (derived from claims data of NHI beneficiaries) was established in 2002 and continues to be maintained for research purposes by Taiwan’s National Health Research Institutes. In this study data, including diagnosis codes, demographic characteristics, and dates of services provided, were available from the NHI research database from different clinical settings (outpatient and inpatient services and emergency department visits). Personal data regarding patients or care providers, including medical institutions and physicians, was pseudonymized; therefore the requirement for informed consent was waived. The institutional review board of the National Taiwan University Hospital approved the study protocol (NTUH-REC number: 201808083W).
Study population
Patients of all ages registered with the Taiwan NHI programme with at least 1 diagnosis code for psoriasis or PsA made by either a dermatologist or a rheumatologist were eligible for this study. The study cohort was identified using the International Classification of Diseases, 9th (ICD-9; used from 2006 to 2015) and 10th (ICD-10; used from 2016 to 2017) editions codes for psoriasis (ICD-9: 696.1; ICD-10: L40.0, L40.4, L40.9) and PsA (ICD-9: 696.0; ICD-10: L40.5, L40.51, L40.52, L40.53, L40.54, L40.59).
To ensure reliable and complete incidence data and avoid misclassification of prevalent cases as incident ones, the lookback period for identifying incident cases was from 2006 and the incidence analysis was limited to the period between 2009 and 2017. In each year, incident cases were defined as patients with a first recorded diagnosis code for psoriasis or PsA. The index date was the date of the first recorded diagnosis, and individuals were considered prevalent cases from that date onwards. Prevalent cases were those with at least 1 diagnosis code before the end of each year. A sensitivity analysis using ≥ 2 diagnosis codes assigned by any medical speciality was also conducted for incidence and prevalence evaluation.
To investigate the associations between psoriasis and PsA and all-cause mortality, each person in the incidence cohort was matched by sex and age on the index date to 10 people without prior history of psoriasis and PsA. The matched cohort was assigned the same index date to ensure that patients with or without psoriasis and PsA were followed up over similar periods. The study cohorts were followed from the index date until death, de-registration from the NHI, or end of the study period (31 December 2017), whichever came first. Furthermore, the matched cohort was censored when they received a psoriasis or PsA diagnosis that was confirmed by a dermatologist or a rheumatologist.
Study outcomes
The annual prevalence of psoriasis and PsA were derived by dividing the number of prevalent cases for each year by all Taiwan NHI registrants in that particular calendar year. The annual incidence of psoriasis and PsA were calculated by dividing the number of incident cases by their person-time at risk. The person-time at risk was calculated from the start of each calendar year or the day of registration of each incident case until the index date, death, or end of each calendar year, whichever came first. Patients with prior codes for psoriasis and PsA were excluded from the denominator and numerator in determining the annual incidence.
Annual mortality rates among incident cases and their matched comparators were derived by dividing the number of deaths occurred in that calendar year by their person-time at risk. The person-time at risk was calculated from the index date until death, de-registration from the NHI, first psoriasis or PsA diagnosis confirmed by a dermatologist or a rheumatologist (for comparators), or end of the calendar year in question, whichever came first.
Statistical analyses
Annual prevalence (percentage and 95% confidence intervals (95% CIs)) and incidence (per 100,000 person-years and 95% CIs) were estimated and stratified by age and sex. Age- and sex-standardized prevalence was estimated by the direct standardization method (14) using the Taiwan population as reference (15).
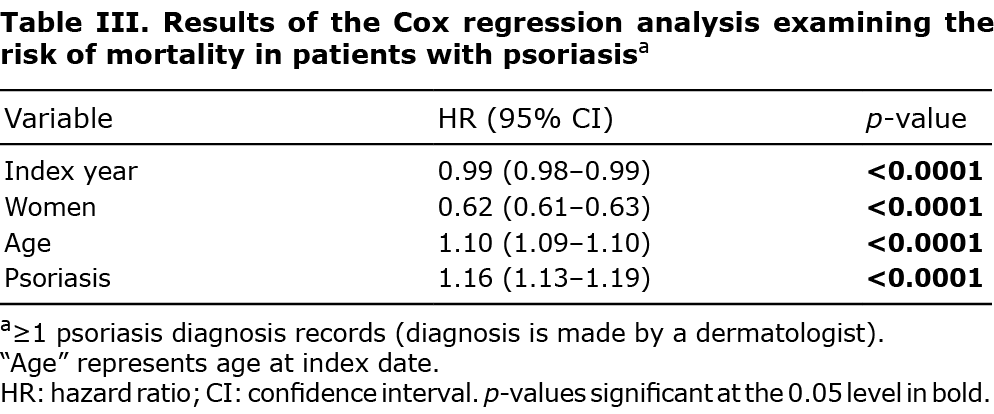
Cox proportional hazards regressions were used to investigate mortality, comparing the patients with psoriasis and PsA with their matched comparators. The regression models included index year (as a continuous variable), sex, and age at index date. The proportional hazards assumption was assessed by examining scatterplots of the model coefficients over time and statistical testing based on weighted residuals. All analyses were carried out using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
During the study period, Taiwan NHI registrants increased from 22,502,536 in 2006 to 23,583,524 in 2017 (Table SI). The number of individuals with psoriasis increased from 40,420 in 2006 to 201,657 in 2017. Overall, 123,303 incident psoriasis cases were diagnosed in 2009 or later (Table I). The number of individuals with PsA included in the study increased from 1,786 in 2006 to 18,209 in 2017. Overall, 12,933 incident PsA cases were diagnosed in 2009 or later (Table I).
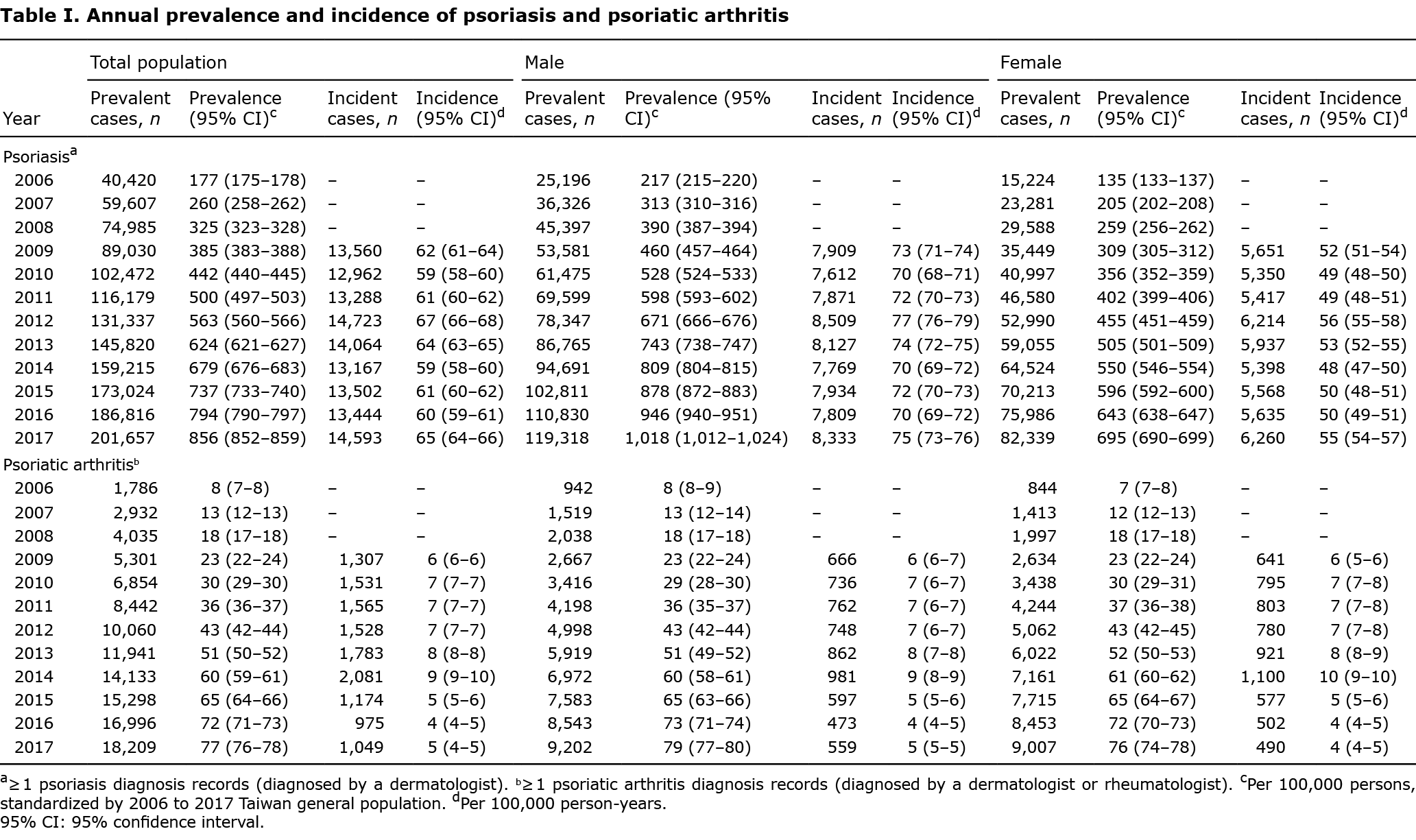
Annual incidence and prevalence of psoriasis
The standardized prevalence rates for clinically diagnosed psoriasis increased steadily, from 0.18% to 0.86% between 2006 and 2017. However, the incidence of psoriasis remained stable between 62 (95% CI 61–64) and 65 (95% CI 64–66) per 100,000 person-years during 2009 and 2017. Overall, the prevalence and incidence of psoriasis were consistently higher in males than females (Table I, Fig. 1).
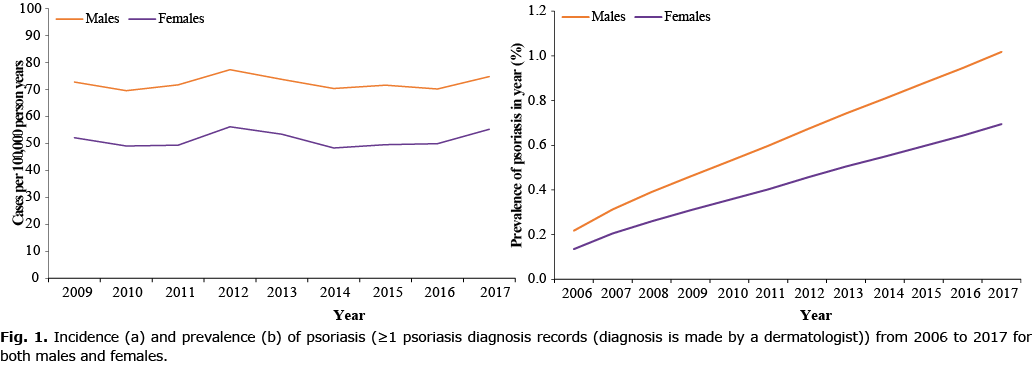
Incidence of psoriasis plotted against age showed a modest bimodal pattern with the frequency of age at first diagnosis peaking at the age of 30–39 years and 80–89 years, characteristic of early-onset (type I) and late-onset (type II), respectively, with a higher proportion of new cases presenting after age 40 years (i.e. late-onset, Fig. 2a). Age-specific incidence rates of psoriasis remained relatively stable within the various age strata from 2009 to 2017 (Fig. 3a), with the incidence among children (0–19 years) much lower compared with adults (Fig. 3a, Table SIII).
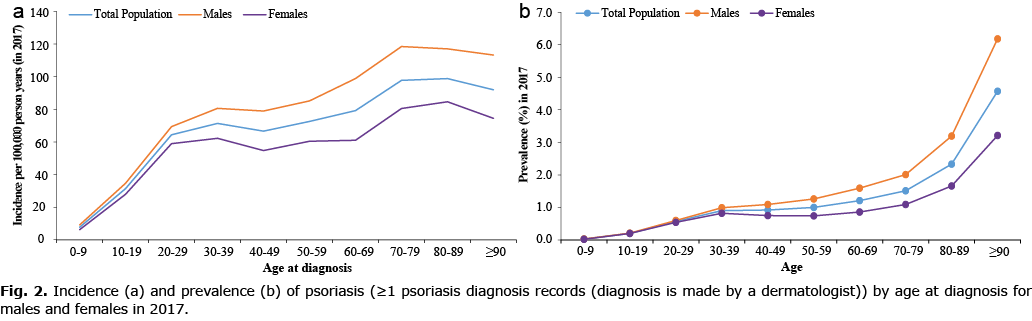
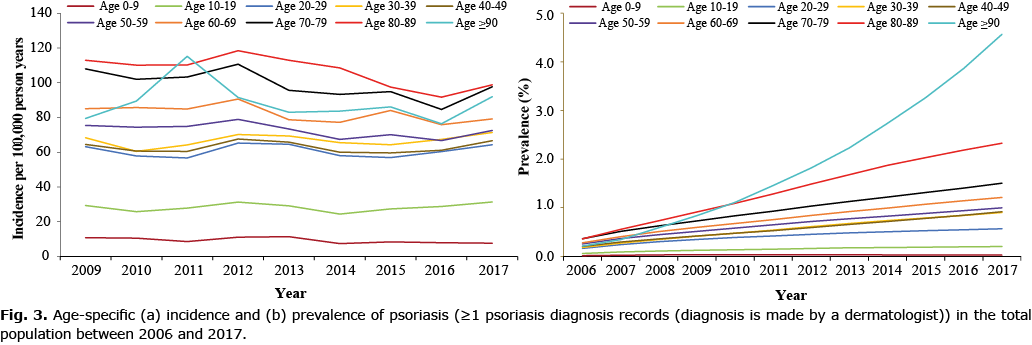
The prevalence of psoriasis increased with age (Fig. 2b). Interestingly, the prevalence was comparable between boys and girls (0–19 years old); however, in adults, the prevalence was much higher in males compared with females (Fig. 2b). Age-specific prevalence rates of psoriasis remained relative stable from 2006 to 2017 among children (0–19 years), but increased steadily in all other age groups particularly among those aged ≥ 90 years old (Fig. 3b, Table SIII).
Similar rates and trends in incidence and prevalence were observed in the sensitivity analysis (Tables SII and SIV).
Annual incidence and prevalence of psoriatic arthritis
The standardized prevalence rates for clinically diagnosed PsA increased steadily from 0.01% to 0.08% between 2006 and 2017. However, the incidence of PsA remained stable between 6 (95% CI 6–6) and 5 (95% CI: 4–5) per 100,000 person-years during 2009 and 2017 (Table I). No difference in the prevalence and incidence of PsA were found between the sexes (Table I, Fig. S1).
Incidence of PsA showed a trend of increasing incidence with age, reaching a peak at the age of 50–59 years, after which it declined (Fig. S2a), while the prevalence of PsA increased with age (Fig. S2b). Interestingly, the prevalence of PsA was comparable between the sexes up to the age of 60–69 years, after which the prevalence was higher in males than females (Fig. S2b). Age-specific prevalence rates of PsA remained relative stable from 2006 to 2017 among children (0–19 years), but increased steadily in all other age groups (Fig. S3b, Table SV).
Similar rates and trends in incidence and prevalence were observed in the sensitivity analysis (Tables SII and SVI).
All-cause mortality in people with psoriasis and psoriatic arthritis compared against matched comparators
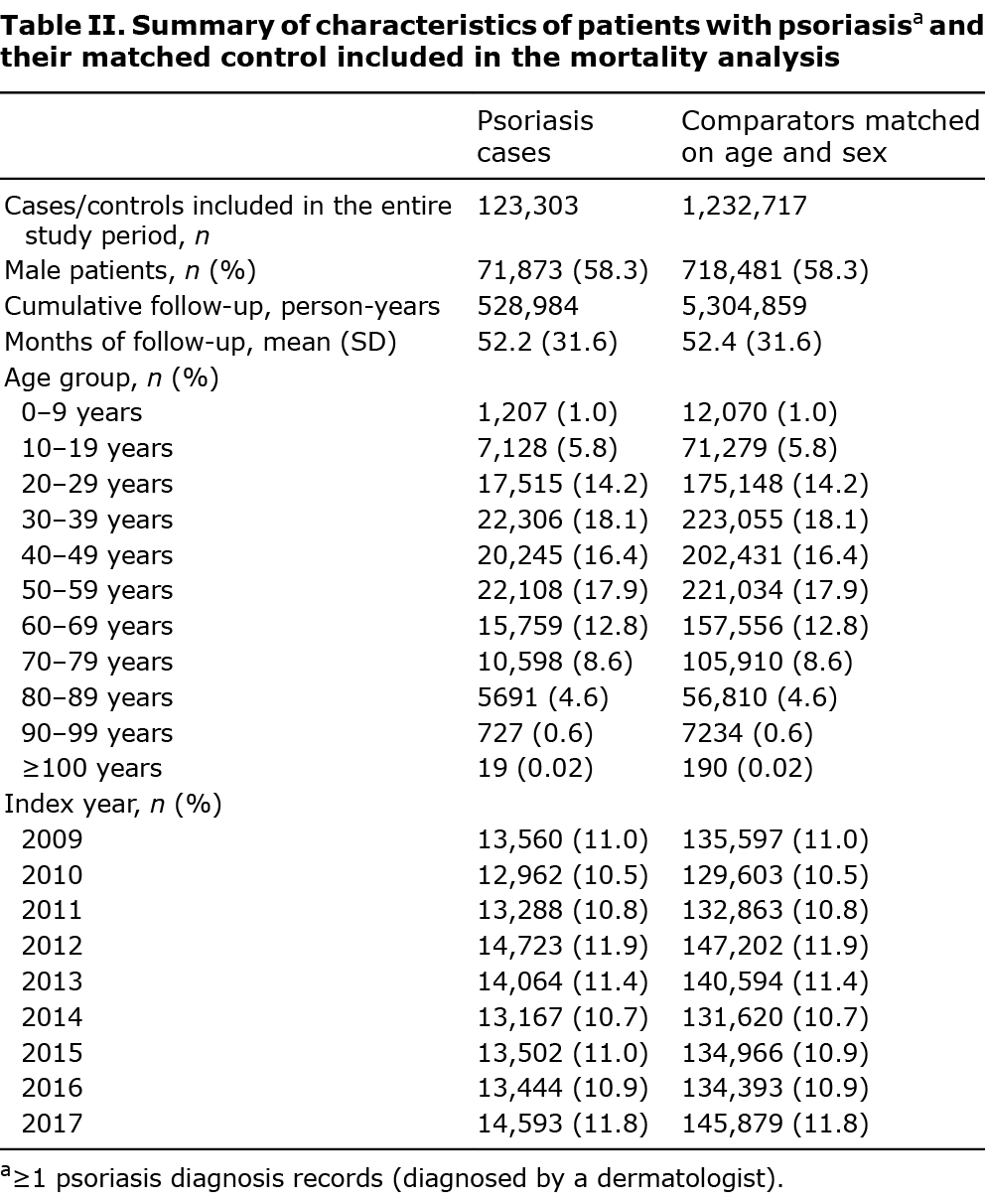
In total, 123,303 people with psoriasis were matched to 1,232,717 comparators between 2009 and 2017. Males accounted for 58% in both cases and controls, and the mean follow-up time (52 ± 32 months) was similar between cases and comparators (Table II). The results of Cox proportional hazards regressions (Table III) showed an overall reduction in mortality over time for all patients (HR 0.99, 95% CI 0.98–0.99, Fig. S4). After controlling for index year, age and sex, patients with psoriasis had a higher mortality risk than the comparators (1.16, 1.13–1.19). Results from the sensitivity analysis were consistent with the findings from the primary analysis (Table SVII). The findings were similar for the association between PsA and all-cause mortality (Tables SVIII, SIX and Fig. S5).
DISCUSSION
This study found the prevalence of clinically diagnosed psoriasis and PsA has increased steadily over 12 years, and the results suggest that psoriasis and PsA affect 0.86% and 0.08%, respectively, of the general population in Taiwan. In contrast, the incidence of psoriasis and PsA remained stable. Age-specific incidence rates remained constant within the various age strata throughout the follow-up period. At the same time, the prevalence increased more steadily in the older age groups than in younger age groups, suggesting an increasing population living longer with psoriasis and PsA. Nevertheless, the risk of all-cause mortality was higher for patients with psoriasis and PsA than in the general population.
An increasing trend in the prevalence of psoriasis and PsA has been observed previously in Taiwan (10, 11) and in several other settings, for example in Korea (16, 17) and Germany (18). This observed increase in the prevalence of psoriasis and PsA in Taiwan has previously been speculated to be attributable to changes in lifestyle and environmental factors (obesity, psychological conditions, and stress), or an increased awareness of the disease among physicians and the general population (possibly due to the advent of biologic therapies) (10). In this study, however, we observed a steady increase in psoriasis and PsA prevalence in the context of a decreasing risk of mortality. As fewer patients die for every incident case over time, we found that the prevalence pool of patients with psoriasis and PsA is increasing steadily. Nevertheless, we found that life expectancy has increased by approximately 3 years and therefore, may not fully explain the observed increase in psoriasis and PsA prevalence. Hence, some of the other reasons that have been highlighted previously to be attributed to the increase in the prevalence of psoriasis and PsA may also help to explain the increase in prevalence observed in our study.
Studies about the longitudinal incidence of psoriasis and PsA in Taiwan are scarce. Similar to our findings, Wei et al. (11) demonstrated that the incidence of psoriasis remained approximately the same between 2000 and 2013. However, in contrast to our findings, they reported that the incidence of PsA increased from 3.64 to 6.91/100,000 person-years between 2000 and 2013. Nevertheless, between 2009 and 2013, the reported incidence rates for PsA remained stable and are similar to the current findings. It is unclear whether the increase in the incidence rate for PsA between 2000 and 2013 represents true changes in PsA incidence over time, changes in the risk factors (obesity, psychological conditions, and stress) for PsA (19), or changes in the diagnosis pattern and awareness of the disease over time (20).
No difference in the incidence and prevalence of psoriasis between boys and girls was observed, but in adults, higher incidence and prevalence rates were observed in men compared with women. These findings align with most of the previous studies that reported a male predominance in East Asia (21–24). The reason for this is not clear (6). Healthcare service utilization is higher among women than men in Taiwan (9, 25); therefore, the possibility that the male predominance in Taiwan might be due to selection bias is unlikely. Nevertheless, societal taboo (for females) and the difference in self-directed health behaviours (e.g. diet, exercise, smoking or alcohol consumption) are some reasons that have been speculated to account for the difference in the incidence and prevalence observed between the sexes, particularly that this difference is observed in adults and not in children (6). Contrary to Wei et al. (11), the current results suggest no difference in the overall incidence and prevalence of PsA between the sexes. Nevertheless, our findings are in line with previous reports on the epidemiology of PsA (26).
Our findings relating to the age of onset of psoriasis, showed a slight bimodal age distribution of psoriasis onset, supporting the notion of “type I” (early-onset) and “type II” (late-onset) variants of the condition. Interestingly, late-onset psoriasis is more common than early-onset type, contrary to the often-quoted convention that 75% of new psoriasis cases present before age 40 years (i.e. early-onset) (27). While the predominance of early-onset psoriasis was confirmed in study populations from dermatology departments, outpatient clinics, and self-reported surveys (28–33); population-based estimates suggested that late-onset psoriasis was more common (34, 35). In addition, the high frequency of late-onset psoriasis among incident cases probably reflects the underlying lower frequency of HLA-Cw6 in the Taiwanese population (36–38). Compared with late-onset psoriasis, HLA-Cw6 is strongly associated with early-onset psoriasis, and its frequency is generally higher in the Caucasian population (27, 37). The discrepancy may also be explained by the underlying population age distribution, with late-onset psoriasis more common in an ageing population (34).
A higher risk of all-cause mortality for patients with psoriasis and PsA compared with the general population was observed in this study. This finding aligns with several previous studies that reported on excess mortality in patients with psoriasis and PsA compared with the general population (12, 39–41). For example, both all-cause mortality and mortality from malignancies and circulatory system diseases have been reported to be elevated in patients with psoriasis and PsA in Taiwan (12). There is growing evidence suggesting a link between psoriasis and other risk factors, which could lead to a higher risk of mortality. Patients with psoriasis are more likely to have cardiovascular disease, metabolic syndrome, obesity, diabetes mellitus, and malignancies, particularly in those with PsA and severe skin disease (40–44), which could explain the higher mortality risk observed in patients with psoriasis. In addition, patients with psoriasis tend to have higher rates of behavioural risk factors, including smoking and alcohol use (45, 46), which could also explain the higher mortality risk observed in patients with psoriasis.
To our knowledge, this is the first study to examine trends in incidence, prevalence and mortality of patients with psoriasis and PsA over a prolonged period among the general population in Taiwan. Although the size of the database and the use of recent data (2006–2017) enabled us to investigate these trends robustly and provide contemporary population-based estimates of disease epidemiology and trends over time, some limitations remain that should be considered alongside the current findings. Firstly, prevalent cases could have been misclassified as an incident if individuals did not seek healthcare services shortly after the onset of symptoms. Nevertheless, restricting the incidence analysis period between 2009 and 2017 allowed ample time for prevalent cases to aggregate in the database. Secondly, due to left censorship the prevalence of psoriasis and PsA may have been underestimated in 2006; however, this is a general limitation for all studies using secondary databases. Thirdly, the actual age of incidence may be systematically lower than our estimates, but we assume such an error would be consistent and not affect the observed trends. Fourthly, the current study only includes those people who seek medical care and thereby receive a diagnosis of psoriasis or PsA, but milder cases who do not present for medical care might be missed. This, however, would also be true in other patient populations and probably, is of lesser magnitude in the current study considering the unique setting of highly accessible healthcare services in Taiwan. Fifthly, we did not adjust for all potential confounders (such as socioeconomic status, lifestyle factors (e.g. smoking and alcohol drinking), environmental factors (e.g. residence – urban, suburban, or rural) and comorbidities) in the regression models used to investigate mortality, as many of these confounders are not routinely captured in the NHI claims database. Finally, we did not analyse prevalence and incidence by disease severity, as standardized measures of severity are not routinely captured in the NHI claims database.
In conclusion, this national cohort study estimated the prevalence and incidence of clinically recognized psoriasis and PsA and mortality rates in Taiwan over 12 years. There was an increase in the prevalence of diagnosed psoriasis and PsA in Taiwan between 2006 and 2017, which does not appear to be attributable to a corresponding increase in incidence. The study found an increasing population living longer with psoriasis and PsA in Taiwan, which contributes to the increasing prevalence of the conditions. Nevertheless, patients with psoriasis and PsA had a higher risk of mortality compared with the general population. These findings have important implications for healthcare services and resource allocation.
ACKNOWLEDGEMENTS
The authors acknowledge the substantial contribution of the Global Psoriasis Atlas (GPA) Project teams at the University of Manchester to the administration of the project. The authors also acknowledge the key role played by the GPA Collaborating Organisations in the establishment and organisation of the GPA: the International Psoriasis Council; the International Federation of Psoriasis Associations; and the International League of Dermatological Societies. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the GPA Collaborating Organisations.
The authors are grateful to Rebekah Swan, GPA Programme Manager.
Data were provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare (project number H108083).
Funding: The Global Psoriasis Atlas has been supported by grants and sponsorships from the LEO Foundation, Abbvie, Celgene, Eli Lilly UK and Company Limited, Janssen, Almirall and Novartis Pharma AG (2019–2020). This project was also funded by National Taiwan University through the Health Data Research Center. The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication. DMA and CEMG are funded in part by the National Institute for Health Research Manchester Biomedical Research Centre.
Study approval: This study received approval from the Institutional Review Board of the National Taiwan University Hospital (NTUH-REC number 201808083W).
Conflicts of interest. KAC has received grants or honoraria from Amgen, Bayer, Boehringer Ingelheim, GlaxoSmithKline, MSD, MundiPharma, and Takeda. CEMG has received honoraria and/or research grants from AbbVie, Almirall, Amgen, BMS, Boehringer Ingelheim, Celgene, Eli Lilly Galderma, LEO Pharma, Janssen, Novartis, Pfizer, Sandoz, Sun Pharmaceuticals, and UCB Pharma. DMA has received grant funding from Abbvie, Almirall, Celgene, Eli Lilly, Janssen, Novartis, UCB and the Leo Foundation. The remaining authors have no conflicts of interest to declare.
REFERENCES
- Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet 2021; 397: 1301–1315.
- Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370: 263–271.
- Springate DA, Parisi R, Kontopantelis E, Reeves D, Griffiths CEM, Ashcroft DM. Incidence, prevalence and mortality of patients with psoriasis: a UK population-based cohort study. Br J Dermatol 2017; 176: 650–658.
- World Health Organization (WHO). Global report on psoriasis. Geneva: WHO, 2016.
- Hsieh C-Y, Su C-C, Shao S-C, Sung S-F, Lin S-J, Yang Y-HK, et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol 2019; 11 349–358.
- Iskandar IYK, Parisi R, Griffiths CEM, Ashcroft DM. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br J Dermatol 2020; 184: 243–258.
- Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ 2020; 369: m1590.
- Chang Y-T, Chen T-J, Liu P-C, Chen Y-C, Chen Y-J, Huang Y-L, et al. Epidemiological study of psoriasis in the national health insurance database in Taiwan. Acta Derm Venereol 2009; 89: 262–266.
- Tsai TF, Wang TS, Hung ST, Tsai PIC, Schenkel B, Zhang M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci 2011; 63: 40–46.
- Wang TS, Hsieh CF, Tsai TF. Epidemiology of psoriatic disease and current treatment patterns from 2003 to 2013: a nationwide, population-based observational study in Taiwan. J Dermatol Sci 2016; 84: 340–345.
- Wei JCC, Shi LH, Huang JY, Wu XF, Wu R, Chiou JY. Epidemiology and medication pattern change of psoriatic diseases in Taiwan from 2000 to 2013: a nationwide, population-based cohort study. J Rheumatol 2018; 45: 385–392.
- Lee MS, Yeh YC, Chang YT, Lai MS. All-cause and cause-specific mortality in patients with psoriasis in Taiwan: a nationwide population-based study. J Invest Dermatol 2017 137: 1468–1473.
- World Health Organization (WHO). 67th World Health Assembly: psoriasis. WHA67.9. Geneva: WHO. 2014.
- Tripepi G, Jager KJ, Dekker FW, Zoccali C. Stratification for confounding – part 2: direct and indirect standardization. Nephron Clin Pract 2010; 116: c322–c325.
- Department of Household Registration Ministry of the Interior Republic of China (Taiwan). Population by sex and 5 year age group for counties and cities.xls. [accessed 2020, March 15] Available at: https://www.ris.gov.tw/app/en/3910.
- Han JH, Lee JH, Han KD, Seo HM, Bang CH, Park YM, et al. Epidemiology and medication trends in patients with psoriasis: a nationwide population-based cohort study from Korea. Acta Derm Venereol 2018; 98: 396–400.
- Lee JY, Kang S, Park JS, Jo SJ. Prevalence of psoriasis in Korea: a population-based epidemiological study using the Korean National Health Insurance Database. Ann Dermatol 2017; 29: 761–767.
- Sewerin P, Brinks R, Schneider M, Haase I, Vordenbaumen S. Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis 2019; 78: 286–287.
- Tollefson MM, Crowson CS, McEvoy MT, Kremers HM. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol 2010; 62: 979–987.
- Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol 2009; 60: 394–401.
- Henan Dermatoses Survey Group. An analysis of 487 cases of psoriasis in 100,000 natural population of Henan rural district. Chin J Dermatol 1982; 15: 83–84.
- Changgeng S, Guowei Z, Guangchao W. Distribution of psoriasis in China: a nationwide screening. Proc Chin Acad Med Sci Peking Union Med Coll 1987; 2: 59–65.
- Ding XL, Wang TL, Shen YW, Wang XY, Zhou C, Tian S, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol 2012; 22: 663–667.
- Li Meijiao, Wang Peng, Cai Min, Liu Qiao, Wu Weiwei, Fu Lei, et al. Prevalence and risk factors of psoriasis in Hainan province: an epidemiological survey. Chin J Dermatol 2013; 46: 157–159.
- Ministry of Health and Welfare. 107 Annual National Health Insurance Medical Statistics Annual Report. [accessed 2020, May 18] Available at: https://dep.mohw.gov.tw/DOS/lp-4648-113.html.
- Ogdie A, Langan S, Love T, Haynes K, Shin D, Seminara N, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford) 2013; 52: 568–575.
- Henseler T, Christophers E. Psoriasis of early and late onset: characterization of two types of psoriasis vulgaris. J Am Acad Dermatol 1985; 13: 450–456.
- Swanbeck G, Inerot A, Martinsson T, Wahlström J, Enerbäck C, Enlund F, et al. Age at onset and different types of psoriasis. Br J Dermatol 1995; 133: 768–773.
- Ferrándiz C, Pujol RM, García-Patos V, Bordas X, Smandía JA. Psoriasis of early and late onset: a clinical and epidemiologic study from Spain. J Am Acad Dermatol 2002 46: 867–873.
- Farber EM, Nall L. The natural history of psoriasis in 5,600 patients. Dermatology 1974; 148: 1–18.
- Youn JI, Park B-S, Park S-B, Kim S-D, Suh D-H. Characterization of early and late onset psoriasis in the Korean population. J Dermatol 1999; 26: 647–652.
- Chularojanamontri L, Kulthanan K, Suthipinittharm P, Jiamton S, Wongpraparut C, Silpa-Archa N, et al. Clinical differences between early- and late-onset psoriasis in Thai patients. Int J Dermatol 2015; 54: 290–294.
- Nevitt GJ, Hutchinson PE. Psoriasis in the community: prevalence, severity and patients’ beliefs and attitudes towards the disease. Br J Dermatol 1996; 135: 533–537.
- Schonmann Y, Ashcroft DM, Iskandar IYK, Parisi R, Sde-Or S, Comaneshter D et al. Incidence and prevalence of psoriasis in Israel between 2011 and 2017. J Eur Acad Dermatol Venereol 2019; 33: 2075–2081.
- Egeberg A, Skov L, Gislason GH, Thyssen JP, Mallbris L. Incidence and prevalence of psoriasis in Denmark. Acta Derm Venereol 2017; 97: 808–812.
- Chiu HY, Huang PY, Jee SH, Hu CY, Chou CT, Chang YT, et al. HLA polymorphism among Chinese patients with chronic plaque psoriasis: subgroup analysis. Br J Dermatol 2011; 166: 288–297.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol 2018; 178: 854–862.
- Chang YT, Tsai SF, Lee DD, Shiao YM, Huang CY, Liu HN, et al. A study of candidate genes for psoriasis near HLA-C in Chinese patients with psoriasis. Br J Dermatol 2003; 148: 418–423.
- Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol 2007; 143: 1493–1499.
- Salahadeen E, Torp-Pedersen C, Gislason G, Hansen PR, Ahlehoff O. Nationwide population-based study of cause-specific death rates in patients with psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 1002–1005.
- Svedbom A, Dalén J, Mamolo C, Cappelleri JC, Mallbris L, Petersson IF, et al. Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol 2015; 95: 809–815.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin 2015; 33: 41–55.
- Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res 2006; 298: 321–328.
- Onumah N, Kircik LH. Psoriasis and its comorbidities. J Drugs Dermatol 2012; 11: s5–s10.
- Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol 2014; 170: 304–314.
- Brenaut E, Horreau C, Pouplard C, Barnetche T, Paul C, Richard MA, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol 2013; 27: 30–35.