ORIGINAL REPORT
Unmet Needs in Darier’s Disease from a Patient’s Perspective: Lessons Learnt from the German Registry
Danielle ROGNER1,2, Laura HEIMERL1, Tilo BIEDERMANN1, Elke SATTLER3 and Alexander ZINK1,4
1Department of Dermatology and Allergy, School of Medicine, Technical University of Munich, Munich, 2Institute for Medical Information Processing, Biometry and Epidemiology (IBE) Faculty of Medicine, LMU Munich Pettenkofer School of Public Health, Munich, 3Department of Dermatology and Allergy, University Hospital, LMU Munich, Munich, Germany, and 4Division of Dermatology and Venereology, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden
The MDHHgermany registry was initiated to characterize the “real-life” situation of affected individuals with Darier’s disease (DD; Morbus Darier, MD) and Hailey-Hailey disease (HH), including their treatment and healthcare. To gain deeper insights into medical care of patients with DD, various aspects such as demographics, subjective symptoms, patient satisfaction with medical care, past and current therapies were explored. Patients with diagnosed DD were included. Subjective symptoms such as itch, pain and burning sensation were assessed. Individual therapy goals were recorded and patients assessed previous/current therapies along with satisfaction of medical care and treatment. A total of 55 patients were recruited; 47 patients were eligible for the analysis. Pruritus was rated the most bothersome symptom. Some 42.6% had not received systemic treatment so far or systemic therapies were rated ineffective (32.6%). Most commonly oral retinoids were prescribed, followed by corticosteroids. Patient satisfaction with medical care and treatment proved to be mediocre. This “real-life” data show an alarming unmet need regarding patients’ satisfaction with medical care and treatment, evidenced by the reported lack of disease control. Further studies and interventions are needed to improve the spectrum of available therapies. MDHHgermany provides a foundational platform for future clinical trials, epidemiological studies, and pathophysiological analyses.
SIGNIFICANCE
Darier’s disease is a rare, autosomal dominantly inherited, debilitating disease, which can lead to stigmatization and social isolation. Its management and therapies remain difficult, as therapeutic options are limited. Aim of our research was to shed light on the in-depth patient’s perspective of the disease and medical care hereof, hopefully enhancing our overall understanding of DD and leading to an improvement of patient management on the whole.
Key words: Darier’s disease; genodermatosis; orphan disease; disease burden; registry.
Citation: Acta Derm Venereol 2024; 104: adv19663. DOI: https://doi.org/10.2340/actadv.v104.19663.
Copyright: © 2024 The Author(s). Published by MJS Publishing, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/).
Submitted: Sep 26, 2023; Accepted after revision: May 13, 2024; Published: Jun 11, 2024
Corr: Dr. med. Danielle Franziska Rogner, Department of Dermatology and Allergy, Technical University of Munich, Biedersteiner Str. 29, DE-80802 Munich, Germany. E-Mail: daniellefranziska.rogner@mri.tum.de
Competing interests and funding: D.R. has received research grants, honoraria as lecturer and/or consultant, and/or travel grants from Eli Lilly, Novartis, and Pfizer. L.H. has no conflicts of interest to be noted. T.B. has no conflicts of interest to be noted. E.S. has no conflicts of interest to be noted. S.H. has no conflicts of interest to be noted. A.Z. has no conflicts of interest to be noted.
Dr Rogner is a La Roche Posay grant recipient (2022). The establishment of the MDHHgermany registry was funded by the Eva Luise und Horst Köhler Stiftung (ELHKS).
INTRODUCTION
Darier’s disease (DD) is a rare genetic disorder characterized by skin lesions and nail abnormalities. The condition follows an autosomal dominant inheritance pattern, caused by mutations in the ATP2A2 gene on chromosome 12q23-24.1, which encodes the sarcoplasmic and endoplasmic reticulum calcium ATPase type 2 (SERCA2) protein (1–4).
The clinical presentation of DD varies in severity and may encompass various skin lesions such as keratotic papules and plaques, typically located on the trunk, scalp, and seborrheic areas. The lesions can coalesce to large papillomatous masses, often accompanied by pruritus and foul odour. In addition, nail abnormalities such as longitudinal striations, subungual hyperkeratosis, and V-shaped notches may be evident (5–7).
The characteristic skin lesions and nail abnormalities associated with the disease can result in physical discomfort, emotional distress, and social stigma, all contributing to a negative impact on patients’ overall well-being (8). To date, 2 studies have demonstrated reduced health-related quality of life (HRQL) in 74 (8) and 133 DD patients (9), respectively. However, the patients’ perspectives on satisfaction with the available medical care and treatments has not been described.
With initiation of the national registry for DD (Morbus Darier, MD) and Hailey-Hailey disease (HHD), MDHHgermany, we aimed to collect data to evaluate the “real-life” situation of healthcare for individuals affected by DD, along with its psychosocial impact (10). This study presents data obtained from an interim analysis on 47 patients with DD included in the registry from June 2020 to March 2023, emphasizing the baseline characteristics combined with the disease severity and the patients’ satisfaction with medical care and treatment.
MATERIAL AND METHODS
MDHHgermany registry
MDHHgermany is a nationwide clinical registry, including patients with DD and HHD from dermatology clinics and office-based dermatologists in Germany (10,11). For objectives see Table SI.
Inclusion criteria for Darier’s disease patients
Individuals who had been clinically and preferably histologically diagnosed with DD and who were either inpatients or outpatients at the selected centres were eligible to participate in the study, provided they were able to understand the German version of the questionnaire. Additionally, we incorporated patients from the self-help platform, as there are several individuals with DD who lack a physician managing their condition. Within the questionnaire, all patients were required to indicate how the diagnosis was made.
Schedule of assessments and study instruments for Darier’s disease patients
Enrolled patients in the registry were followed up prospectively for a minimum of 24 months, throughout which paper-based questionnaires were filled out by both doctors and patients, or alternatively online using REDCap (Research Electronic Data Capture; REDCap 12.2.2, © 2022 Vanderbilt University) (12). For paper-based questionnaires, the data were manually entered into the REDCap system by the dermatology department of the Technical University of Munich.
Patient registry questionnaires were completed at inclusion (baseline visit; V1) and then ideally every 6 months using REDCap. Unscheduled visits were possible if required due to flare-ups or if desired by the patient for other reasons.
To achieve high acceptance and unbiased free-text answers, we incorporated innovative questionnaires in addition to validated questionnaires including the numeric rating scale (NRS) (13) and DLQI (see Table SII) (8). At baseline (V1), the patient was requested to complete a questionnaire encompassing 5 sections, covering basic demographic details, familial and personal medical history, the overall severity of the disease, specific symptoms, and recent therapies and treatment objectives, as well as questions regarding the psychosocial impact (Table SII).
To record clinical data in addition to subjective data and improve patient satisfaction, physician study visits were conducted at patient inclusion (V1) and ideally once a year or after any changes in treatment. These visits were not performed for patients who had participated via the self-help platform and include information on current and past therapies, reasons for treatment decisions made by the physician, as well as an assessment of the severity of DD (using the proposed “DD score” by Amar et al. in a recent publication) (14).
Data management and statistical analysis
All assessments were electronically documented using CE-certified software solutions (ESPRIO, Seracom Software Solutions GmbH, Stuttgart, Germany and REDCap, Research Electronic Data Capture; REDCap 8.5.28, ©2019 Vanderbilt University, Nashville, TN, USA). For this interim analysis, only baseline data included in the registry between 10 June 2020 and 15 March 2023 were considered. Prior to analysis, data were checked for plausibility and completeness. Incomplete questionnaires, in which less than 75% of all questions were answered, were deemed ineligible and excluded from the study. In cases where the disease was not explicitly specified in the questionnaire, all responses provided by that particular patient were disregarded. When calculating a score, the presence of a single missing value in any question resulted in the exclusion of all answers for the purpose of score calculation. However, when analysing individual questions within an assessment, the patient’s response was still considered. The entire analysis was based on descriptive statistics performed in R studio version 2023.3.0.386 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographics and general patient characteristics
In total 55 patients with DD were enrolled in the registry, and 47 patients were analysed in this first data interim analysis. The remaining (n = 8) participants were excluded due to missing and/or implausible data. The patients’ demographic data including family history, education, and working capability is described in Table I. At inclusion, the mean age of the study population was 49.4 ± 14.4 years, with females accounting for 67.4% of the cohort.
The majority (80.9%) of the study population were employed. Interestingly, 25.5% of all patients were on sick leave for 1–10 days throughout the past year due to DD.
Some 57.9% of DD patients knew of affected family members (on mean 2.5 ± 1.2 affected family members). It was assumed by the patients that the severity of the family members’ disease ranged from moderate to severe for 39.3%.
Disease history, activity, and doctor’s consultation before inclusion in the registry
The mean age at first diagnosis was 24.1 ± 12.1 with 21 being diagnosed before the age of 20 (Fig. 1), as commonly stated in the literature (1). The mean disease duration before registry inclusion was 25.1 ± 17.3 years (Table II).
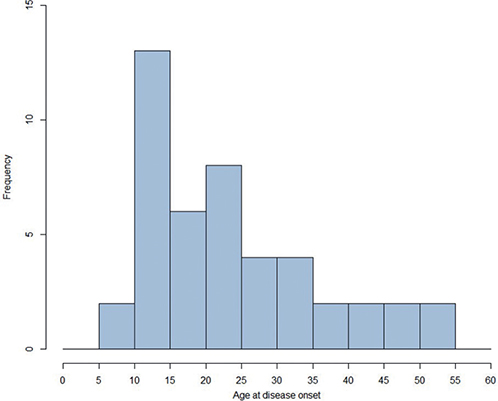
Fig. 1. Age at first diagnosis (n = 47) (min: 6 years, max: 53 years). x-axis: age in numbers.
When evaluating the intensity of their own condition (mild, moderate, or severe), 86.4% opted for the classifications of “moderate” or “severe” (Fig. 2) and the majority (58.7%) noted the presence of persistent DD lesions over the past year (see Table II).
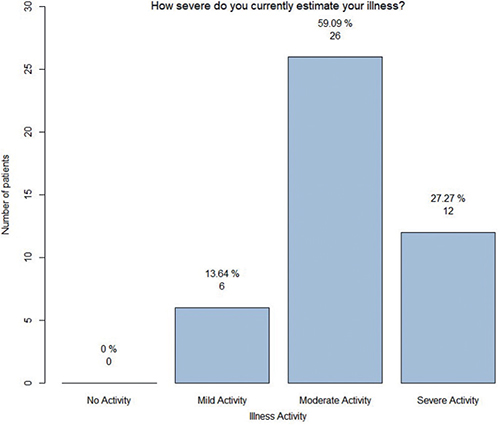
Fig. 2. Display of assessment of disease severity (n = 44). y-axis: number of patients.
In considering the requirement for healthcare of DD patients, it is noteworthy that, on average, the patients had 1.4 ± 2.4 inpatient stays in the past 5 years and 4.9 ± 4.4 outpatient stays in the past 12 months. Interestingly, 45.5% reported a slight improvement in the skin condition upon an inpatient stay, while 18.2% noted that the skin condition had worsened.
As expected, the most consulted specialist was a dermatologist (93.0%), followed by general practitioner (34.9%); 14.9% of the patients had seen a naturopath in the past 5 years.
The predominant reasons for consultations were the deterioration of DD (83.7%), prescription of medication (48.8%), and routine check-ups regarding DD (41.9%). Overall, patients stated having spent €49.7 ± 50.7 on their disease per month for non-healthcare plan medication (see Table II).
Subjective symptoms and disease burden regarding Darier’s disease
Participants assessed pain, itch, burning sensation, and sleeplessness over the past 3 days on a numeric rating scale ranging from 0–10, whereby 0 reflects not being troubled at all and 10 indicates being very troubled (13). Pruritus was rated with the highest value (5.6 ± 2.7), followed by burning sensation (3.9 ± 2.7), pain (3.6 ± 3.0), and sleep disorder (3.2 ± 3.2) (Table III, Figs 3 and 4). Accordingly, when patients were asked to select from a variety of aspects which pose the highest burden on them, pruritus was chosen by most patients (n = 37), followed by lower self-esteem and pain (Fig. 5).
| Assessment | |
| Consulted doctor explains reasons for therapy recommendation, n (%) | n = 43, NA = 4 |
| Doesn’t apply at all | 8 (18.6) |
| Applies slightly | 4 (09.3) |
| Applies partly | 13 (30.2) |
| Applies mostly | 5 (11.6) |
| Applies fully | 13 (30.2) |
| Consulted doctor includes me and my therapeutic goals in decisions, n (%) | n = 43, NA = 4 |
| Doesn’t apply at all | 3 (7.0) |
| Applies slightly | 10 (23.3) |
| Applies partly | 10 (23.3) |
| Applies mostly | 7 (16.3) |
| Applies fully | 13 (30.2) |
| Consulted doctor gives me a chance to participate in the decision with regard to the therapy, n (%) | n = 42, NA = 5 |
| Doesn’t apply at all | 5 (11.9) |
| Applies slightly | 6 (14.3) |
| Applies partly | 5 (11.9) |
| Applies mostly | 13 (31.0) |
| Applies fully | 13 (31.0) |
| Assessment | |
| How satisfied are you currently (last 3 months) with the medical care (caretaking by your physician etc.) of your skin disease?a | 44, NA = 3 |
| Mean ± SD | 4.1 ± 3.2 |
| How satisfied are you currently (last 3 months) with the medical treatment (systemic and topical therapies) of your skin disease?a | 43, NA = 4 |
| Mean ± SD | 3.7 ± 3.1 |
| Patient’s report on (in the past 3 days) | 45, NA = 2 |
| Pruritus (0- to 10-point scale)b, mean ± SD | 5.6 ± 2.7 |
| Pain (0- to 10-point scale)b, mean ± SD | 3.6 ± 3.0 |
| Sleep disorder (0- to 10-point scale)c, mean ± SD | 3.2 ± 3.2 |
| Burning sensation (0- to 10-point scale), mean ± SDd | 3.9 ± 2.7 |
| a0 = very dissatisfied, 10 = very satisfied; 0- to 10-point scale. b0 = no pain; 10 = worst imaginable pain. c0 = no disturbance; 10 = worst disturbance. d0 = no burning sensation; 10 = worst imaginable burning sensation. SD: standard deviation; NA: . |
|
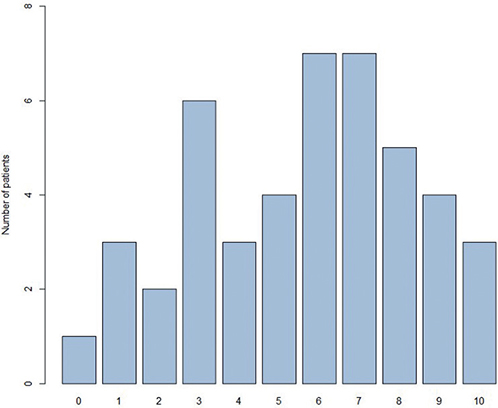
Fig. 3. Display of the symptom “itch” (n = 45) on a 10-point-scale, 0 = no itch, 10 = worst imaginable itch. x-axis: 0- to-10 point scale; y-axis: number of patients.
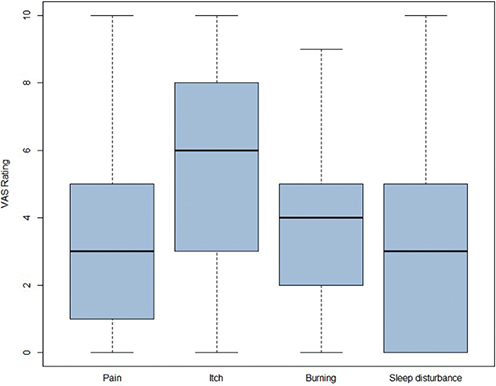
Fig. 4. Bar plot of subjective symptoms pain, itch, burning sensation, and sleep disturbance during the past 3 days (n = 45). y-axis: 0- to-10 point scale.
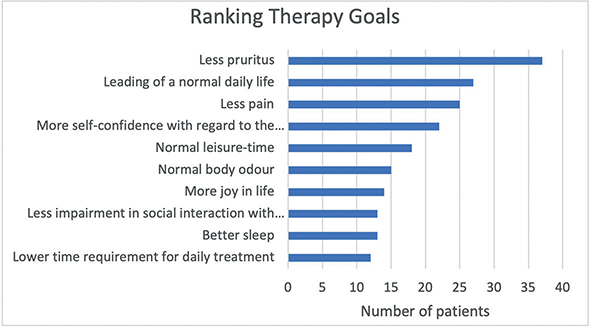
Fig. 5. Display of ranking of disease burden (n = 46). x-axis: number of patients.
Frequency and effectiveness of available therapies
Almost all included patients (n = 44) received topical therapy, while only 27 patients received systemic medication. Topical corticosteroids (97.7%) emerged as the primary prescribed topical medication, followed by topical retinoids (32.6%), whereas the most prescribed systemic medication was retinoids (66.7%), especially acitretin, followed by systemic corticosteroids (59.3%) (Table IV). Concerning effectiveness of topical therapy, 44.2% of patients rated topical steroids as the most efficacious. However, 32.6% reported ineffectiveness of any topical treatment (Table IV). In accordance, 29.6% rated retinoids the most effective systemic therapy, and 11 (40.7%) found no systemic therapy effective at all (Table IV).
Satisfaction with medical care and therapeutic goals
To assess the patients’ perspectives on medical care, the questionnaire evaluated their inclusion in therapy decisions and satisfaction levels. Only 30.2% of patients described doctors having fully explained therapeutic recommendations, while 18.6% received no explanation. Accordingly, 30.2% experienced full inclusion in decision-making, 16.3% mostly, 23.3% partly, and 23.3% slightly. The remaining patients did not feel involved.
On a 0- to 10-point satisfaction scale (0 = dissatisfied, 10 = very satisfied) for medical care and treatment in the last 3 months, the mean values were 4.1 ± 3.2 and 3.7 ± 3.1, respectively, indicating mild to moderate dissatisfaction (see Table IV).
Concerning therapy goals (Fig. 6), a vast majority (80.4%) priorixtized ending pruritus, followed by achieving a normal daily life (58.7%) and eliminating pain (54.3%).
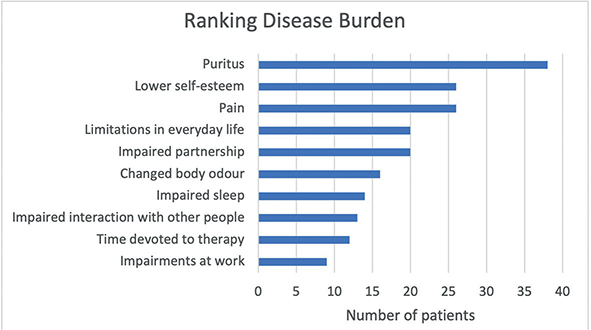
Fig. 6. Display of therapeutic goals, more than one answer possible (n = 46). x-axis: number of patients.
DISCUSSION
Darier’s disease (DD) frequently proves to be a debilitating condition that significantly affects the quality of life and, to date, remains difficult to treat (9).
As already anticipated when this registry was started, our initial interim analysis supported 2 main observations: (a) individuals impacted by DD are not receiving adequate treatment, as evidenced by their dissatisfaction with medical care, and (b) the disease activity remains high, contributing to a moderate to severe disease burden, likely attributed to the reported lack of effective therapeutic options.
One of the most important findings was that the majority of patients rated the general satisfaction with medical care and treatment below mediocre. A considerable number of individuals reported ineffectiveness of both external and systemic therapies. In addition to the patients’ personal perspective, the insufficient efficacy was also evident by the amount of in- and outpatient stays, potentially imposing a considerable burden on the health system.
The baseline characteristics could show that a special focus of future research should be brought to the burden inflicted by pruritus, which constantly emerged as the most distressing symptom across various tools. In accordance, its alleviation was ranked the primary therapeutic goal for patients in the MDHHregistry, indicating that available therapies are not sufficient to reduce itch. Unfortunately, despite a single successful case report in the literature, conventional antihistamines do not demonstrate a significant impact when used in the day-to-day clinical routine, altogether underlining the pressing need for efficient antipruritic treatments (15).
Apart from the relevance of pruritus and pain management, the registry also revealed that altered body odour affected the life of DD patients. It is noteworthy that a potential therapeutic strategy could emerge from novel findings by Amar et al. (14). The authors highlighted the role of dysbiosis in odour changes and demonstrated potential management through microbiome transplantation or targeted enhancement of beneficial bacteria. In particular, S. hominis and C. acnes could improve the disease activity by mitigating inflammation and body odours linked to exacerbations.
Our finding that most patients consistently experienced skin issues throughout the year, coupled with an alarming 86.4% who categorized their disease activity as “moderate” or “severe”, further supports the notion that DD still requires more efficient therapies due to a lack of adequate disease control. Overall, we show that despite our progress in understanding the underlying pathophysiology of DD (1, 16, 17), the development of new therapies has not succeeded during the last decades. Strikingly, we still rely on the same therapies that were already employed 20 years ago. To that end, retinoids remain the most effective and commonly prescribed, even though the side effects and the teratogenicity often leave us with hands tied (18, 19). While the literature describes numerous case reports on various therapies (2,20), none has yet achieved the required breakthrough (2). In contrast, registries like MDHHgermany can provide the first step to explore unconventional therapy options that have proved successful in several patients, forming the basis for broader clinical trials of new treatment regimens and the improvement of existing therapies.
Limitations
Our data analysis comes with several limitations, above all the size of the study cohort, as numerous centres did not have capacity to undergo the time-consuming ethics process. Therefore, the majority of patients were recruited in Bavaria. Nevertheless, DD is a rare disease and affected patients have never been assessed this extensively. Furthermore, the subjective symptoms burn, pruritus, sleep, and burning were assessed only for the past 3 days, introducing a potential bias as patients tend to consult a doctor when the disease activity is at its peak. Consequently, we also faced a potential selection bias for severely affected and already diagnosed patients, as individuals who regularly visit in- or outpatient departments or actively searched for health information regarding DD were more likely to participate in the registry. Lastly, the assessment of current and past therapies did not differentiate between topical and systemic medication during a flare-up and medication when the skin is stable, which is, however, crucial in our everyday routine.
It is important to note that our analysis relies primarily on the patients’ perspective, and assessments like disease severity were not validated by a direct comparison with a physician’s clinical assessment. Additionally, some participants entered the registry without a physician (especially from the self-help platform), hence the diagnosis of DD is not entirely definite. However, the primary goal of this analysis was to assess the patients’ perspective rather than objective clinical data. The correlation of patients’ and physicians’ assessments within MDHHgermany will be examined in the future.
Conclusion
In conclusion, these baseline characteristics offer valuable insights into the current healthcare landscape and treatment approaches for patients with DD in Germany. Our “real-life” data could demonstrate an alarming burden inflicted by the disease on the patients and reveal a critical shortage of efficient therapies, emphasizing the urgent need for additional agents to achieve a perceptible improvement of the disease activity in DD patients. We found that treatment strategies should aim at alleviating the most burdensome symptoms, especially pruritus, ideally tailored to the patient’s individual needs and preferences.
We hope that this “real-life” data collection will enhance physicians’ overall understanding of their DD patients, guide physicians’ decision-making regarding therapeutic goals and contribute to improved management of these difficult-to-treat patients.
ACKNOWLEDGEMENTS
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Ethics statement: The registry has formally been approved by the local ethics committee of the Technical University of Munich (49/19 S-SR). All participating sites handed in the ethics at their local ethics committee.
REFERENCES
- Cooper SM, Burge SM. Darier’s disease: epidemiology, pathophysiology, and management. Am J Clin Dermatol 2003; 4: 97–105.
- Rogner DF, Lammer J, Zink A, Hamm H. Darier and Hailey-Hailey disease: update 2021. J Dtsch Dermatol Ges 2021; 19: 1478–1501.
- Miyauchi Y, Daiho T, Yamasaki K, Takahashi H, Ishida-Yamamoto A, Danko S, et al. Comprehensive analysis of expression and function of 51 sarco(endo)plasmic reticulum Ca2+-ATPase mutants associated with Darier disease. J Biol Chem 2006; 281: 22882–22895.
- Sakuntabhai A, Dhitavat J, Burge S, Hovnanian A. Mosaicism for ATP2A2 mutations causes segmental Darier’s disease. J Invest Dermatol 2000; 115: 1144–1147.
- Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol 1992; 27: 40–50.
- Holzberg M BR, de Berker D, Thomas L. Chapter 6: The nail in dermatological disease. Hoboken, NJ: Wiley; 2012.
- Munro CS. The phenotype of Darier’s disease: penetrance and expressivity in adults and children. Br J Dermatol 1992; 127: 126–130.
- Dodiuk-Gad R, Cohen-Barak E, Ziv M, Shani-Adir A, Amichai B, Zlotogorski A, et al. Health-related quality of life among Darier’s disease patients. J Eur Acad Dermatol Venereol 2013; 27: 51–56.
- Harris A, Burge SM, Dykes PJ, Finlay AY. Handicap in Darier’s disease and Hailey-Hailey disease. Br J Dermatol 1996; 135: 959–963.
- Rogner DZ, A. MDHHgermany. p. https://www.derma-allergie.med.tum.de/fuer-patienten/darier-und-hailey-hailey.html.
- Rogner D, Heimerl L, Heyer S, Biedermann T, Sattler E, Zink A. Patients’ perspective, quality of life and treatment goals in Hailey-Hailey disease: Lessons learned from the German National Registry. J Eur Acad Dermatol Venereol 2024; 38: 419–429.
- Lyon JA, Garcia-Milian R, Norton HF, Tennant MR. The use of Research Electronic Data Capture (REDCap) software to create a database of librarian-mediated literature searches. Med Ref Serv Q 2014; 33: 241–252.
- Weisshaar E, Bentz P, Apfelbacher C, Haufe E, Heinrich L, Heratizadeh A, et al. Itching in atopic dermatitis: patient- and physician-reported outcomes in the German Atopic Dermatitis Registry TREATgermany. Acta Derm Venereol 2023; 103: adv00854.
- Amar Y, Rogner D, Silva RL, Foesel BU, Ud-Dean M, Lagkouvardos I, et al. Darier’s disease exhibits a unique cutaneous microbial dysbiosis associated with inflammation and body malodour. Microbiome 2023; 11: 162.
- Hsieh CY, Tsai TF. Use of H-1 antihistamine in dermatology: more than itch and urticaria control: a systematic review. Dermatol Ther (Heidelb) 2021; 11: 719–732.
- Savignac M, Simon M, Edir A, Guibbal L, Hovnanian A. SERCA2 dysfunction in Darier disease causes endoplasmic reticulum stress and impaired cell-to-cell adhesion strength: rescue by Miglustat. J Invest Dermatol 2014; 134: 1961–1970.
- Zhao Q, Fu F, Fu X, Wang Z, Liu H, Zhang F. Three novel mutations of the ATP2A2 gene in Chinese patients with Darier disease. Australas J Dermatol 2019; 60: e171–e172.
- Hulatt L, Burge S. Darier’s disease: hopes and challenges. J R Soc Med 2003; 96: 439–441.
- Vieira ML, de Paula Samorano L, da Matta Rivitti-Machado MC, de Oliveira ZNP. Darier disease: long-term treatment with systemic retinoids at a tertiary hospital. J Dtsch Dermatol Ges 2020; 18: 628–630.
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol 2021; 87: 14–21.