SHORT COMMUNICATION
Adrenal Insufficiency after Long-term use of Topical Glucocorticoids in Patients with Advanced Cutaneous T-cell Lymphomas: A Case Series
Valerie GLUTSCH1#, Patrick SCHUMMER1#, Ann-Cathrin KOSCHKER2, Matthias GOEBELER1 and Marion WOBSER1*
1Department of Dermatology, Venereology and Allergology, and 2Endocrinology and Diabetes Unit, Department of Medicine I, University Hospital Würzburg, Josef-Schneider-Straße 2, DE-97080 Würzburg, Germany. *E-mail: Wobser_M@ukw.de
Citation: Acta Derm Venereol 2024; 104: adv19672. DOI https://doi.org/10.2340/actadv.v104.19672.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/)
Submitted: Sep 27, 2023; Accepted: Mar 4, 2024; Published: Apr 2, 2024
Competing interests and funding: The authors have no conflicts of interest to declare.
VG and PS are supported by the Else Kröner Forschungskolleg TWINSIGHT and by the Interdisziplinäres Zentrum für klinische Forschung (IZKF), University Hospital Würzburg.
INTRODUCTION
Cutaneous T-cell lymphomas (CTCLs) are rare haematopoietic neoplasms that are confined to the skin at the time of diagnosis (1). Especially in the early stages, skin-directed therapies, e.g. topical glucocorticoids (TGCs), chlormethine gel, UV-light treatment, or localized radiation, represent the central treatment pillar (1). However, in later stages, which are primarily treated with systemic therapies, TGCs are usually continued to treat skin manifestations. It is well known that TGCs can be absorbed percutaneously, especially depending on the period of application, the amount and potency, potential alterations in skin barrier or body surface area (BSA) treated (2). Thus, systemic effects of TGCs such as steroid-induced diabetes mellitus or Cushing’s syndrome have already been documented in various inflammatory dermatoses (3,4). In CTCLs, these side effects have not yet been systematically recorded. As CTCLs often require prolonged and extensive TGC use over years to decades, it is likely that the risk of percutaneous absorption is increased in these patients. Here, we report 3 cases of clinically asymptomatic adrenocortical insufficiency due to TGC use in patients with advanced CTCL.
CASE REPORT
The first case is a male patient who was diagnosed with mycosis fungoides (MF). He received numerous different local and systemic therapies mostly accompanied by treatment with TGCs. The period of intermittent TGC use thus amounted to more than 20 years. Due to progressive disease to tumour stage under low-dose gemcitabine therapy, 12 cycles of mogamulizumab were applied. Lesional use of TGC was continued (Fig. 1a and b). A routine blood check revealed a significantly reduced level of cortisol without adequate increase in adrenocorticotropic hormone (ACTH) (Table I). An abnormal ACTH stimulation test supported the suspected adrenal insufficiency. However, our patient did not show any symptoms of cortisol deficiency, putatively owing to continued TGC treatment. After reviewing all findings and excluding hypophysitis by MR imaging in addition to further endocrinological laboratory testing, we diagnosed iatrogenic tertiary adrenocortical insufficiency due to long-term use of TGCs.
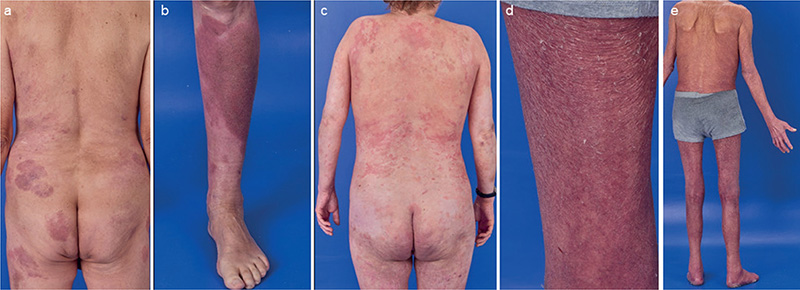
Fig. 1. Representative skin lesions at diagnosis of adrenal insufficiency. (a–b) Case 1: Sharply demarcated erythematous, discretely scaly plaques on the back, gluteal area, and the lower leg treated with mometasone furoate creme once daily. (c) Case 2: Disseminated elevated and erythematous patches and plaques on the entire integument treated with mometasone furoate creme once daily. (d–e) Case 3: Pachyerythroderma treated with clobetasol propionate ointment twice daily.
| Case 1 | Case 2 | Case 3 | |
| Age at first diagnosis, years | 41 | 35 | 72 |
| CTCL type | MF | MF | SS |
| CTCL stage (ISCL/EORTC 2007 [11,12]) at diagnosis of adrenal insufficiency | IIB | IVA2 | IVA1 |
| Years of TGC use | >20 | 9 | 5 |
| Potency of TGC applied at time of diagnosis of adrenal insufficiency | High potency | High potency | Superpotent |
| TGC applied at time of diagnosis of adrenal insufficiency | Mometasone furoate | Mometasone furoate | Clobetasol propionate |
| Application frequency of TGC, daily | 1x | 1x | 2x |
| BSA treated with TGC, % | 20 | 80 | 80 |
| Basal cortisol at diagnosis of adrenal insufficiency, µg/dL (normal range 5–25 µg/dL) | 1.6 | <1 | 2 |
| ACTH at diagnosis of adrenal insufficiency, ng/L (normal range 0–46 ng/L) | 8.0 | <5 | 5.5 |
| CTCL: cutaneous T-cell lymphoma; TGC: topical glucocorticoids; MF: mycosis fungoides; SS: Sézary syndrome; BSA: body surface area; ACTH: adrenocorticotropic hormone. | |||
Case 2 is a female patient who was diagnosed with MF. She received various systemic treatments, each accompanied by an intermittent application of high-potent TGCs. In total, this resulted in 9 years of TGC use. Beside mild dermatoporosis and telangiectasias, no steroid-related skin toxicity was evident (Fig. 1c). A routine blood check under mogamulizumab treatment revealed a significantly reduced cortisol level (Table I). She reported some increased fatigue in the last months. However, these symptoms were difficult to interpret due to her medical history with piloid astrocytoma, previous CNS irradiation, and mental retardation. After exclusion of pituitary insufficiency due to irradiation and partial resection of previously known astrocytoma, we diagnosed iatrogenic tertiary adrenocortical insufficiency as a result of long-term use of TGCs.
The third case is a male patient who was diagnosed with Sézary syndrome. Beside extracorporeal photopheresis he had received methotrexate and bexarotene as systemic treatments with continuous application of TGCs on large body surface areas for 5 years. Mainly due to pachyderma and erythroderma, no skin toxicity of TGC application was evident (Fig. 1d and e). Upon pre-screening for mogamulizumab treatment, significantly lowered levels of cortisol were detected without clinical symptoms of an adrenal crisis. An adrenocortical insufficiency was confirmed by an ACTH stimulation test. Hence, we diagnosed iatrogenic tertiary adrenocortical insufficiency due to long-term use of TGCs.
Patient demographics and information on topical therapy are summarized in Table I. All patients received substitution with oral hydrocortisone: cases #2 and #3 as daily medication, case #1 as stand-by emergency treatment in case of symptoms. All patients were informed about the dimensions of the disease and potential aggravation factors, as well as proper emergency treatment upon acute symptomatic adrenal insufficiency in addition to continued TGC application.
DISCUSSION
The application of TGCs represents an important backbone for multimodal treatment in dermatological practice, not only for the treatment of inflammatory dermatoses but also in CTCLs (5). Apart from skin adverse effects, systemic absorption and thus systemic side effects may occur with long-term and extensive use. These include the development of adrenal insufficiency alongside symptoms such as nausea, vomiting, hypotension, fatigue, and hyponatremia among others (6,7). While response rates to traditional systemic therapies (e.g. bexarotene, methotrexate) have been mostly moderate in CTCLs, newer therapies such as the anti-CD30 antibody brentuximab vedotin and anti-CCR4 antibody mogamulizumab can achieve rapid and long-lasting remissions and symptom reduction (8,9). This suggests that a TGC therapy previously carried out for years may be discontinued quickly after start of these treatments, owing to rapid response. However, abrupt discontinuation of TGCs is of clinical relevance if adrenal insufficiency has already been induced. In our patients, TGCs have been continuously applied for at least 5 years. In our patients, the additional use of TGCs was consistently indicated despite intensive systemic therapy due to treatment-reluctant skin manifestations. Therefore, there was no need for an abrupt termination of TGCs, which could have led to the clinical manifestation of an acute adrenal crisis.
In the absence of clinical symptoms, the diagnosis of cortisol deficiency is challenging because determination of cortisol and ACTH levels is usually not included in standard laboratory monitoring of systemically treated CTCL. In patients undergoing immunotherapies, however, endocrinological laboratory tests are performed regularly as these treatments are known to trigger endocrinopathies (10). Two of our patients were treated with mogamulizumab at the time as adrenal insufficiency was detected. Hence, the anti-CCR4 antibody was also taken into account as possible trigger of hypophysitis as known from immunotherapies such as checkpoint inhibitors. However, so far there is no described case of mogamulizumab-associated hypophysitis or adrenal insufficiency, and laboratory testing ruled out insufficiency of other hypothalamic–pituitary axes, making hypophysitis less probable. In addition, our patients have been extensively treated with potent TGCs for years (7). Hence, after taking all findings into account, the diagnosis of iatrogenic tertiary adrenocortical insufficiency due to the long-term use of TGCs was plausible in all of our cases.
Our case series has several limitations. The main limitation is the small number of patients and the non-systematic data collection. A systematic analysis of CTCL patients with many years of extensive use of high-potent TGCs would probably find more cases and may be addressed in collaborative projects in the future.
In conclusion, CTCL patients are at risk of developing iatrogenic tertiary adrenal insufficiency. This finding is of immediate clinical impact because abrupt cessation of TGC treatment may induce life-threatening adrenal crisis in such a scenario. In the light of novel systemic therapies such as brentuximab vedotin and mogamulizumab being capable of achieving rapid skin response in CTCL – thus going along with no need for further TGC application – this is especially relevant for the management of CTCL patients.
ACKNOWLEDGEMENT
Publication of patient photos was approved by written consent.
REFERENCES
- Dippel E, Assaf C, Becker JC, von Bergwelt-Baildon M, Bernreiter S, Cozzio A, et al. S2k-Guidelines – Cutaneous lymphomas (ICD10 C82 - C86): Update 2021. J Dtsch Dermatol Ges 2022; 20: 537–554.
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006; 54: 1–15; quiz 16–18.
- Phan K, Smith SD. Topical corticosteroids and risk of diabetes mellitus: systematic review and meta-analysis. J Dermatolog Treat 2021; 32: 345–349.
- Tempark T, Phatarakijnirund V, Chatproedprai S, Watcharasindhu S, Supornsilchai V, Wananukul S. Exogenous Cushing’s syndrome due to topical corticosteroid application: case report and review literature. Endocrine 2010; 38: 328–334.
- Nguyen CV, Bohjanen KA. Skin-directed therapies in cutaneous T-cell lymphoma. Dermatol Clin 2015; 33: 683–696.
- Bockle BC, Jara D, Nindl W, Aberer W, Sepp NT. Adrenal insufficiency as a result of long-term misuse of topical corticosteroids. Dermatology 2014; 228: 289–293.
- Levin C, Maibach HI. Topical corticosteroid-induced adrenocortical insufficiency: clinical implications. Am J Clin Dermatol 2002; 3: 141–147.
- Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 2017; 390: 555–566.
- Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol 2018; 19: 1192–1204.
- Gonzalez-Rodriguez E, Rodriguez-Abreu D, Spanish Group for Cancer I-B. Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist 2016; 21: 804–816.
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019; 133: 1703–1714.
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110: 1713–1722.