ORIGINAL REPORT
Contact Allergy to Allergens in the Swedish Baseline Series Overrepresented in Diabetes Patients with Skin Reactions to Medical Devices: A Retrospective Study from Southern Sweden
Josefin ULRIKSDOTTER1,2, Thanisorn SUKAKUL1, Magnus BRUZE1, Martin MOWITZ1, Robert OFENLOCH3 and Cecilia SVEDMAN1
1Department of Occupational and Environmental Dermatology, Lund University, Skåne University Hospital, Malmö, Sweden, 2Department of Dermatology, Helsingborg Hospital, Helsingborg, Sweden and 3Occupational Dermatology, Department of Dermatology, University Hospital Heidelberg, Heidelberg, Germany
Allergic contact dermatitis is reported among individuals using continuous glucose monitoring systems and insulin pumps. The aim of this study was to describe contact allergy patterns for allergens in the Swedish baseline series and medical device-related allergens among users. Contact allergy to baseline series allergens and isobornyl acrylate was compared between diabetes patients and dermatitis patients patch-tested at the Department of Occupational and Environmental Dermatology during 2017 to 2020. Fifty- four diabetes patients and 2,567 dermatitis patients were included. The prevalence of contact allergy to fragrance mix II and sesquiterpene lactone mix was significantly higher in diabetes patients compared with dermatitis patients. Of the diabetes patients 13.0% and of the dermatitis patients 0.5% tested positive to sesquiterpene lactone mix (p < 0.001). Of the diabetes patients 7.4% and of the dermatitis patients 2.3% tested positive to fragrance mix II (p = 0.041). Of the diabetes patients 70.4% tested positive to medical device-related allergens. Of the diabetes patients 63.0% and of the dermatitis patients 0.2% were allergic to isobornyl acrylate (p < 0.001). In conclusion, not only medical device-related contact allergies, but also contact allergy to baseline series allergens (fragrance mix II and sesquiterpene lactone mix), is overrepresented in diabetes patients who use medical devices.
Key words: allergic contact dermatitis; continuous glucose monitoring; diabetes type 1; insulin pump; isobornyl acrylate; patch-testing.
SIGNIFICANCE
This study shows an overrepresentation of contact allergy to allergens in the Swedish baseline series (fragrance mix II and sesquiterpene lactone mix) among individuals with diabetes using medical devices. The cause of this overrepresentation of contact allergy to allergens not traditionally associated with the use of medical devices needs to be further elucidated. Preventing further exposure is important to avoid new cases of contact allergy among users as well as to avoid the elicitation of allergic contact dermatitis among sensitized individuals.
Citation: Acta Derm Venereol 2024; 104: adv19676. DOI https://doi.org/10.2340/actadv.v104.19676.
Copyright: © Published by Medical Journals Sweden, on behalf of the Society for Publication of Acta Dermato-Venereologica. This is an Open Access article distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
Submitted: Sep 27, 2023; Accepted: Jan 23, 2024; Published: Mar 29, 2024
Corr: Josefin Ulriksdotter, Department of Occupational and Environmental Dermatology, Skåne University Hospital, Jan Waldenströms gata 18, SE-205 02 Malmö, Sweden. E-mail: Josefin.ulriksdotter@med.lu.se
Competing interests and funding: The Gorthon Foundation (Gorthon Stiftelsen), Hudfonden (an organization under 3 foundations in Sweden: Edvard Welanders Stiftelse, Finsenstiftelsen, Fundraising Foundation Hudfonden for Swedish dermatological research), Svenska Diabetesstiftelsen, Asthma and Allergy Association Research Fund (Astma- och Allergiförbundetsforskningsfond).
The study was approved by the Swedish Ethical Review Authority (dnr 2020-02190).
MB is a member of the expert panel for fragrance safety (http://fragrancesafetypanel.org/). CS participates in the fragrance study Extended Fragrance Ingredients Surveillance Study (EFISS) performed on behalf of the The International Fragrance Association (IFRA).
INTRODUCTION
In recent years, many cases of contact allergy to substances found in medical devices (MDs), particularly continuous glucose monitoring (CGM) systems and insulin pumps, have been reported. To date, isobornyl acrylate (IBOA) has been a main allergen (1–6). Among IBOA-allergic patients using FreeStyle Libre, a higher-than-expected number of patients has been reported to be sensitized to fragrances and sesquiterpene lactone mix (SLM) (7, 8). However, the underlying reason for the concomitant positive reactions is not known, and neither fragrances nor SLM constituents have been identified in the glucose sensors.
MDs are not ingredient-labelled; thus their content is largely unknown. Topical products used to prepare skin sites for the MDs, device removal and treatment of skin reactions also pose potential risks of contact sensitization among users. Knowledge of the contact allergy pattern among users exposed to allergens in MDs and related products is essential for preventive measures.
The aim of the current study is to report the contact allergy rates and patterns seen in diabetes patients patch-tested due to suspected allergic contact dermatitis (ACD) to CGM systems and/or insulin pumps, compared with consecutive dermatitis patients patch-tested because of suspected ACD.
MATERIALS AND METHODS
Patients and patch-testing
Adult patients referred to and patch-tested due to dermatitis between October 2017 and October 2020 at the Department of Occupational and Environmental Dermatology in Malmö, Sweden (YMDA) or its branch clinics were included. Patch-test results for some of the diabetes patients have been published in detail previously as case reports (5, 9–13). Patients’ characteristics, including age, sex, and a history of atopic dermatitis (AD) were recorded.
Diabetes patients patch-tested due to suspected ACD to their CGM systems and/or insulin pumps were grouped as “diabetes patients”. Other patients patch-tested with the department’s MD series, such as patients with ostomy, were excluded. “Dermatitis patients” refer to all other patients included (Fig. 1). The study was approved by the Swedish Ethical Review Authority (dnr 2020-02190).
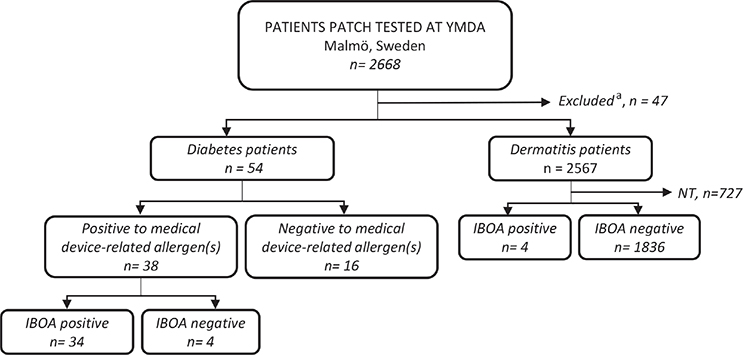
Fig. 1. Flowchart of patients included. IBOA: isobornyl acrylate; n: number of patients; NT: not tested with IBOA; YMDA: Department of Occupational and Environmental Dermatology, Malmö, Sweden. aPatients patch-tested with the department’s medical device (MD)-series who were not diabetes patients.
To also assess the potential importance of IBOA as an allergen in other patient groups it has been patch-tested in consecutive dermatitis patients at YMDA at concentrations of 0.1% and 0.3% w/w since 2018 and 2020, respectively (14).
Patch-test results for allergens in the Swedish baseline series (Table II), different variants of the department’s MD series (Table III) and for IBOA were retrieved from the department’s patch-test registers.
| Substance, concentration (%) & vehicle | Diabetes patients | Dermatitis patients | p-value | ||
| Contact allergy D3–4 or 7 n (%) | Tested n | Contact allergy D3–4 or 7 n (%) | Tested n | ||
| Swedish baseline series | |||||
| Potassium dichromate, 0.5 petrolatum | 1 (1.9) | 54 | 99 (3.9) | 2,557 | 0.72 |
| p-Phenylene diamine, 1.0 petrolatum | 0 | 54 | 69 (2.7) | 2,531 | 0.40 |
| Thiuram mix, 1.0 petrolatum | 0 | 54 | 36 (1.4) | 2,559 | > 0.99 |
| Neomycin sulphate, 20.0 petrolatum | 0 | 54 | 17 (0.7) | 2,561 | > 0.99 |
| Cobalt chloride hexahydrate, 0.5 petrolatum | 3 (5.6) | 54 | 99 (3.9) | 2,555 | 0.47 |
| Quaternium 15, 1.0 petrolatum | 0 | 54 | 20 (0.8) | 2,562 | > 0.99 |
| Nickel(II)sulphate hexahydrate, 5.0 petrolatum | 8 (14.8) | 54 | 414 (16.3) | 2,537 | 0.77 |
| Quinoline mix, 6.0 petrolatum | 1 (1.9) | 54 | 13 (0.5) | 2,562 | 0.25 |
| Colophonium 20.0 petrolatum | 2 (3.7) | 54 | 64 (2.5) | 2,557 | 0.40 |
| Paraben mix, 16.0 petrolatum | 0 | 54 | 9 (0.4) | 2,562 | > 0.99 |
| Black rubber mix, 0.6 petrolatum | 1 (1.9) | 54 | 13 (0.5) | 2,561 | 0.25 |
| Sesquiterpene lactone mix, 0.1 petrolatum | 7 (13.0) | 54 | 13 (0.5) | 2,560 | < 0.001a |
| Mercapto mix, 2.0 petrolatum | 0 | 54 | 3 (0.1) | 2,562 | > 0.99 |
| Epoxy resin, 1.0 petrolatum | 0 | 54 | 29 (1.1) | 2,558 | > 0.99 |
| Myroxylon pereirae, 25.0 petrolatum | 7 (13.0) | 54 | 168 (6.6) | 2,556 | 0.063 |
| p-tert-Butylphenol formaldehyde resin, 1.0 petrolatum | 0 | 54 | 20 (0.8) | 2,561 | > 0.99 |
| Fragrance mix II, 14.0 petrolatum | 4 (7.4) | 54 | 60 (2.3) | 2,561 | 0.041b |
| Formaldehyde, 2.0 aq. | 1 (1.9) | 54 | 89 (3.5) | 2,559 | > 0.99 |
| Fragrance mix I, 8.0 petrolatum | 2 (3.7) | 54 | 156 (6.1) | 2,558 | 0.77 |
| Phenol formaldehyde resin, 1.0 petrolatum | 0 | 54 | 19 (0.7) | 2,561 | > 0.99 |
| Diazolidinyl urea, 2.0 aq. | 0 | 54 | 10 (0.4) | 2,561 | > 0.99 |
| MCI/MI, 0.02 aq. | 1 (1.9) | 54 | 95 (3.7) | 2,555 | 0.72 |
| Amerchol L 101, 50.0 petrolatum | 0 | 54 | 15 (0.6) | 2,562 | > 0.99 |
| Caine mix II, 10.0 petrolatum | 1 (1.9) | 54 | 30 (1.2) | 2,559 | 0.48 |
| Lichen acid mix, 0.3 petrolatum | 1 (1.9) | 54 | 18 (0.7) | 2,561 | 0.33 |
| Tixocortal-21-pivalate, 0.1 petrolatum | 1 (1.9) | 54 | 30 (1.2) | 2,562 | 0.48 |
| Textile dye mix, 6.6 petrolatum | 1 (1.9) | 54 | 75 (2.9) | 2,556 | > 0.99 |
| Budesonide, 0.01 petrolatum | 1 (1.9) | 54 | 16 (0.6) | 2,562 | 0.30 |
| Methyldibromo glutaronitrile, 0.5 petrolatum | 2 (3.7) | 54 | 77 (3.0) | 2,562 | 0.68 |
| Methylisothiazolinone, 0.2 aq. | 4 (7.4) | 54 | 97 (3.8) | 2,554 | 0.15 |
| Extended baseline series | |||||
| Isobornyl acrylatec | 34 (63.0) | 54 | 4 (0.2) | 1,840 | < 0.001d |
| Allergen groups | |||||
| Fragrancese | 11 (20.4) | 54 | 336 (13.2) | 2,552 | 0.123 |
| Metalsf | 11 (20.4) | 54 | 525 (20.7) | 2,535 | 0.951 |
| Preservativesg | 5 (9.3) | 54 | 253 (9.9) | 2,553 | 0.874 |
| Rubbersh | 1 (1.9) | 54 | 48 (1.9) | 2,558 | > 0.999 |
| aOdds ratio (OR)(95% confidence interval [CI]) 29.41 (11.11–76.92). bOR(95% CI) 3.33 (1.17–9.52). cPatch-tested with at least 1 concentration of isobornyl acrylate patch-test preparations. dOR (95% CI) 780.03 (253.10–2403.85). eColophonium, fragrance mix I, II, lichen acid mix, and Myroxylon pereirae resin. fNickel(II)sulphate hexahydrate, cobalt(II)chloride hexahydrate, and potassium dichromate. gMethylchloroisothiazolinone/methyl isothiazolinone (MCI/MI), methyl isothiazolinone (MI), formaldehyde, paraben mix, diazolidinyl urea, methyldibromoglutaronitrile (MDBGN), and quarternium 15. hMercapto mix, black rubber mix, and thiuram mix. Aq: aqua; D: patch-test reading day; n: number of patients with positive reactions. |
|||||
| Version October 2017 Substances and patch-test concentration (%) | Version July 2020 Substances and patch-test concentration (%) | Manufacturer |
| 2-Hydroxyethyl acrylate, 0.1 | 2-Hydroxyethyl acrylate, 0.1 | C |
| Urethane diacrylate, aliphatic, 0.1 | Urethane diacrylate, aliphatic, 0.1 | C |
| Isobornyl acrylate, 0.01, 0.1 | Isobornyl acrylate, 0.01, 0.1a, 0.3a | S-Ab |
| 2,4-Di-tert-butylphenol, 1.0 | 2,4-Di-tert-butylphenol, 1.0 | S-Ab |
| Butylated hydroxytoluene (BHT), 2.0 | Butylated hydroxytoluene (BHT), 2.0 | C |
| Urethane dimethacrylate, 2.0 | Urethane dimethacrylate, 2.0 | C |
| Isophorone diisocyanate, 1.0 | Isophorone diisocyanate, 1.0 | C |
| Isophorone diamine, 0.1 | Isophorone diamine, 0.1 | C |
| 4-tert-Butylphenol, 1.0 | 4-tert-Butylphenol, 1.0 | C |
| N,N-Dimethylacrylamide, 0.1 | N,N-Dimethylacrylamide, 0.1, 0.3 | S-Ab |
| Ethyl cyanoacrylate, 5.0 | C | |
| Alantolactonec, 0.1 | S-Ab | |
| Costunolidec, 0.1 | S-Ab | |
| Dehydrocostus lactonec, 0.1 | S-Ab | |
| Tetrahydrofurfuryl acrylate, 0.1 | S-Ab | |
| 1,6-Hexanediol diacrylate, 0.1 | C | |
| Ethyl acrylate, 0.1 | C | |
| 2-Phenoxyethyl acrylate, 0.1 | Pb | |
| 2-Carboxyethylacrylate, 0.1 | S-Ab | |
| Hydroabietyl alcohol, 10.0 | C | |
| Abietic acid, 10.0 | C | |
| Colophoniumd, 60.0 | S-Ab | |
| N-Vinylcaprolactam, 1.0 | S-Ab | |
| 2-Ethylhexyl acrylate, 0.1 | C | |
| Dipropylene glycol diacrylate, 0.1 | Tb | |
| 2,2’-Methylenebis(6-tert-butyl-4-methylphenol), 1.0 | Tb | |
| Dilauryl thiodipropionate, 1.0 | S-Ab | |
| Lauryl acrylate, 0.1 | S-Ab | |
| 2,2’-Methylenebis(6-tert-butyl-4-methylphenol) monoacrylate, 0.1e | CTAb | |
| aTested in the department’s extended baseline series. bTest preparation prepared in-house. cVehicle ethanol (Kemetyl, Haninge, Sweden). dVehicle softisan. eVarious concentrations from 0.1% and higher. A: Acros Organics, Geel, Belgium; C: Chemotechnique Diagnostics, Vellinge, Sweden; CTA: Chemtronica AB, Sollentuna, Sweden; EtOH: ethanol (Kemetyl, Haninge, Sweden); P: Polysciences, Inc. Warrington, Pennsylvania; pet.: petrolatum (vaselin, vitt; APL, Stockholm, Sweden): used in all pet. preparations not delivered by C; S-A: Sigma-Aldrich, Steinheim, Germany; T: TCI Europe N.V., Zwijndrecht, Belgium. Vehicle petrolatum unless specified. |
||
The term “MD-related allergens” refers to allergens included in different variants of “the Department’s MD series”, IBOA 0.1% and 0.3% in petrolatum (pet.) and to colophonium 20% in pet., respectively. The term “fragrance allergens” refers to the fragrance allergens in the Swedish baseline series (colophonium, fragrance mix (FM) I, II, lichen acid mix, and Myroxylon pereirae resin (MP)). The term “preservative allergens” refer to methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), methylisothiazolinone (MI), formaldehyde, paraben mix, diazolidinyl urea, methyldibromoglutaronitrile (MDBGN), and quarternium 15. “Metal allergens” refer to nickel(II)sulphate hexahydrate, cobalt(II)chloride hexahydrate, and potassium dichromate and “rubber allergens” refer to mercapto mix, black rubber mix, and thiuram mix (Table II).
The allergens (Chemotechnique MB Diagnostics AB, Vellinge, Sweden, and for the MD-related allergens also in house preparations (11)) were tested by applying 20 mg petrolatum preparations (40 mg/cm²) and 15 μL liquid preparations (30 μL/cm²) in either 8-mm Finn Chambers or 8-mm Finn Chambers Aqua (SmartPractice, Phoenix, AZ, USA), or 25 mg petrolatum preparations (39 mg/cm²) and 20 μL (31 μL/cm²) liquid preparations in IQ Ultra or IQ Ultimate chambers (Chemotechnique MB Diagnostics AB). Patch-test chambers were occluded on the subject’s back for 48 h. Patch-test reading is at YMDA, performed on day (D)3 or D4 and D7. The tests were read and scored according to the International Contact Dermatitis Research Group and European Society of Contact Dermatitis criteria (15, 16).
Statistical analysis
Statistical analysis was performed using PASW Statistics for Windows (version 23.0; SPSS Inc., Chicago, IL, USA). The clinical data and prevalence of positive reactions to allergens and allergen groups were calculated using a descriptive statistical analysis. Numbers and percentages are reported. Weak (1+), strong (2+), and extreme (3+) positive reactions were grouped as positive. Irritant, negative, and doubtful reactions were grouped as negative. The proportion of patients with contact allergy to allergen in the Swedish baseline series and IBOA was compared between diabetes patients and dermatitis patients. Among the diabetes patients, the proportion of patients with positive reactions to the allergens in the Swedish baseline series was compared between IBOA-positive and IBOA-negative individuals. Two-sided Pearson’s χ2 test or Fisher’s exact test was performed to compare the proportion of patients and number of reactions in 2 different groups. Fisher’s exact test was applied when the sample size was small (1 or more expected values are less than 5). When the mean age was compared for 2 groups, independent t-test was used. A p-value of less than 0.05 was considered statistically significant. Detailed positive reactions to MD-related allergens were also assessed and concomitant positive reactions to other allergens in IBOA-positive patients are reported.
RESULTS
In total, 54 diabetes patients and 2,567 dermatitis patients were included. In Table I, demographic data are shown. Diabetes patients were significantly less likely to have AD compared with dermatitis patients (13.0% compared with 28.1%, p = 0.014).
Comparison of contact allergies in diabetes patients and dermatitis patients
The proportions of diabetes patients and dermatitis patients with positive reactions to the selected allergens are summarized in Table II. Among the diabetes patients 13.0% were allergic to SLM, compared with 0.5% among the dermatitis patients (p < 0.001). Among the diabetes patients 7.4% were allergic to FM II, compared with 2.3% among the dermatitis patients (p = 0.041). The proportion of patients with contact allergy to fragrance group of allergens was higher among the diabetes patients compared with the dermatitis patients, although the difference was not statistically significant. Almost two-thirds of the diabetes patients (63.0%) were positive to IBOA compared with 0.2% of the dermatitis patients (p < 0.001).
Comparison of contact allergies in IBOA-positive and IBOA-negative diabetes patients
Among the diabetes patients, 52.9% of IBOA-positive patients and 40.0% of IBOA-negative patients had at least 1 simultaneous reaction to allergens in the Swedish baseline series (p = 0.36). Seven of 34 (20.6%) IBOA-positive diabetes patients and none of 20 IBOA-negative diabetes patients were allergic to SLM (p = 0.038). For each of the other allergens in the Swedish baseline series, no significant difference in the proportion of patients with positive reactions was seen among IBOA-positive compared with IBOA-negative diabetes patients. All 4 FM II allergic diabetes patients were also allergic to IBOA; however, the difference in the proportion of FM II allergic patients among IBOA-positive compared with IBOA-negative diabetes patients was not significant (p = 0.29). In total, 8 of 34 (23.5%) IBOA-positive diabetes patients and 3 of 20 (15%) IBOA-negative diabetes patients were allergic to at least 1 fragrance allergen (p = 0.510).
Contact allergy to other medical device-related allergens
In 38 of 54 diabetes patients (70.4%), contact allergy to MD-related allergens was found. In 4 of the 38 individuals contact allergy to IBOA was not seen. In these 4 individuals contact allergy to the following MD-related allergens was seen; isophorone diisocyanate (individual 1), N,N-dimethylacrylamide (individual 2), 2,4-di-tert-butylphenol and butylated hydroxytoluene (BHT) (individual 3), and colophonium (individual 4).
DISCUSSION
Contact allergy to MDs was initially related to IBOA in a single glucose sensor (6). Today, many culprit allergens have been identified in different MDs. The current study found an overrepresentation of contact allergy also to baseline series allergen not traditionally associated with the use of MDs (FM II and sesquiterpene lactones (SLs)) among diabetes patients using CGM and insulin pumps. This makes the magnitude of the problem and the implications for those affected far greater than initially expected.
Overrepresentation of contact allergy to sesquiterpene lactones and fragrance mix II
Contact allergy to FM II was overrepresented in the diabetes patients compared with the dermatitis patients (p = 0.041; Table II). The prevalence of contact allergy to MP was higher among the diabetes patients compared with the dermatitis patients, although the difference was not statistically significant (p = 0.063). Both the diabetes patients and the dermatitis patients have dermatitis, and therefore a damaged skin barrier locally, where exposure to fragrances in scented leave-on and rinse-off products can lead to fragrance contact allergy. However, since contact allergy to FM II was overrepresented in the diabetes patients compared with the dermatitis patients it is likely that the diabetes patients are exposed to FM II from their MDs or related products. Previously, d-limonene has been found in both colophonium-containing and colophonium-free adhesives (tapes/dressings) used by patients with contact allergy to hydroperoxides of limonene, suggesting that the contact allergy could be related to exposure to adhesives in MDs (21). However, the presence of hydroperoxides of limonene in the adhesives has not been confirmed.
Contact allergy to fragrances and SLs has also been found to be overrepresented in patients with AD compared with those without AD (22–24). However, as the prevalence of AD was lower among the adult diabetes compared with dermatitis patients in this study, this cannot explain the higher prevalence of contact allergy to SLM and FM II among the diabetes patients.
Low frequency of IBOA allergy among consecutive dermatitis patients
The prevalence of IBOA allergy was significantly higher among the diabetes patients compared with the dermatitis patients. IBOA has been widely used in high concentrations in glues in MDs (1–4, 6). However, it is also used in other adhesives/glues, coatings, sealants, paints, and inks. From that point of view, the sensitization rate (0.2%) in the dermatitis patients was lower than expected. One of the 4 dermatitis patients sensitized to IBOA had a history of skin reactions to her insulin pump, which was not the reason for the contact allergy investigation. In the second IBOA-positive dermatitis patient a possible relevant exposure was found as she had positive reactions to different nail polishes, which may contain IBOA. In the other IBOA-positive dermatitis patients no relevant exposure could be found. In previous studies sensitization to IBOA patch-tested at 0.1% concentration was also rare in general dermatitis patients (25, 26). Even though sensitization to IBOA is rare in general dermatitis patients, it has proven a major sensitizer when used in MDs, such as CGMs and insulin pumps, where exposure is prolonged.
Concomitant positive reactions in IBOA-allergic diabetes patients
In line with previous studies (7, 8, 11) SLM contact allergy was overrepresented among IBOA-allergic diabetes patients. Notably, a recent study (27) reported stronger patch-test reactions to SLM when retesting at the site of a previous positive patch-test reaction to IBOA, suggesting cross-reactivity between the substances. A possible explanation could be rotation of single bonds, allowing IBOA to present itself in a conformation that can mimic the α-methylene-γ-butyrolactone ring that is present in SLs (27).
A previous study (8) has reported a high proportion of IBOA-sensitized patients (11 of 18; 61%) to be co-sensitized to fragrance allergens. In the current study, contact allergy to FM II and fragrance allergens in general was not significantly more common in IBOA-positive diabetes patients compared with IBOA-negative diabetes patients. However, all 4 FM II allergic patients were IBOA-allergic; hence a significant difference between IBOA-positive and IBOA-negative individuals might have been seen in a larger patient population.
Contact allergy to medical device-related allergens over time
Most of the diabetes patients were allergic to MD-related allergens, indicating that their skin reactions were a manifestation of ACD. The composition of the MDs changes over time and in different batches (9), hence correct diagnosis of the skin reactions and relevance assessment of contact allergies is challenging. During the study period the contact allergy rates for IBOA and N,N-dimethylacrylamide (DMAA) (63.0% and 24.2% (unpublished data), respectively) were high among the diabetes patients. Cases of contact allergy to these substances have been reported (6, 13) and efforts have been made by the manufacturers to remove these allergen from their products (28, 29). In patients investigated at YMDA during the period November 2020 to June 2022 the contact allergy frequencies for IBOA and DMAA decreased to 23.7% and 2.6%, respectively (unpublished data). Simultaneously, new allergens, namely dipropylene glycol diacrylate (DPGDA) and 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate were identified in MDs (9, 10), at YMDA. High contact allergy frequencies were seen for DPGDA (21.1%) and 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (31.6%) in diabetes patients patch-tested due to suspected ACD to MDs during the period November 2020 to June 2022 (unpublished data). Replacing known allergens in the products is an important, but delicate, task with a risk of introducing yet new allergens. Continuous chemical analyses of the products, relevant and up-to-date MD patch-test series, and case reports of contact allergy to new allergens are important for prevention of contact allergy to substances in MDs. In 2017, our department used an MD patch-test series with 11 substances. Further substances have been included and patch-test concentrations adjusted (Table III) (2, 5, 7, 9, 10, 12, 13, 30). To-date, the series consists of 34 different patch-test preparations, and further modifications will be made continuously based on new information on sensitizers in MDs.
Conlusion
Targetted patch-testing with IBOA, other relevant MD-related allergens, the product, and extracts thereof, is necessary when ACD to MDs is suspected. However, IBOA cannot presently be recommended as a screening allergen in general dermatitis patients. As colophonium is a MD-related allergen and contact allergy to SLs and FM II was overrepresented among the diabetes patients with ACD to MDs, baseline series allergen should also be patch-tested. Contact allergy to SLM is related to IBOA allergy, while no such association was found for FM II.
REFERENCES
- Raison-Peyron N, Mowitz M, Bonardel N, Aerts O, Bruze M. Allergic contact dermatitis caused by isobornyl acrylate in OmniPod, an innovative tubeless insulin pump. Contact Dermatitis 2018; 79: 76–80.
- Herman A, Baeck M, de Montjoye L, Bruze M, Giertz E, Goossens A, et al. Allergic contact dermatitis caused by isobornyl acrylate in the Enlite glucose sensor and the Paradigm MiniMed Quick-set insulin infusion set. Contact Dermatitis 2019; 81: 432–437.
- Malinauskiene L, Slekyte G, Mowitz M, Isaksson M, Zablockis R. Allergic contact dermatitis caused by isobornyl acrylate in two patients treated for idiopathic pulmonary arterial hypertension. Contact Dermatitis 2020; 83: 170–171.
- Renaudin H, Darrigade AS, Dendooven E, Foubert K, Aerts O, Milpied B. Allergic contact dermatitis from a disposable blood pressure cuff containing isobornyl acrylate and 2-phenoxyethyl acrylate. Contact Dermatitis 2021; 84: 462–464.
- Hamnerius N, Mowitz M. Intense skin reaction to a new glucose monitoring and insulin pump system. Contact Dermatitis 2020; 83: 524–527.
- Herman A, Aerts O, Baeck M, Bruze M, De Block C, Goossens A, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle(R) Libre, a newly introduced glucose sensor. Contact Dermatitis 2017; 77: 367–373.
- Herman A, Mowitz M, Aerts O, Pyl J, de Montjoye L, Goossens A, et al. Unexpected positive patch test reactions to sesquiterpene lactones in patients sensitized to the glucose sensor FreeStyle Libre. Contact Dermatitis 2019; 81: 354–367.
- Dendooven E, Foubert K, Goossens A, Gilles P, De Borggraeve W, Pieters L, et al. Concomitant positive patch test reactions in FreeStyle-allergic patients sensitized to isobornyl acrylate. Contact Dermatitis 2021; 84: 166–174.
- Ulriksdotter J, Svedman C, Bruze M, Mowitz M. Allergic contact dermatitis caused by dipropylene glycol diacrylate in the Omnipod® insulin pump. Br J Dermatol 2022; 186: 334–340.
- Svedman C, Ulriksdotter J, Lejding T, Bruze M, Mowitz M. Changes in adhesive ingredients in continuous glucose monitoring systems may induce new contact allergy pattern. Contact Dermatitis 2021; 84: 439–446.
- Ulriksdotter J, Svedman C, Bruze M, Glimsjö J, Källberg K, Sukakul T, et al. Contact dermatitis caused by glucose sensors – 15 adult patients tested with a medical device patch test series. Contact Dermatitis 2020; 83: 301–309.
- Svedman C, Bruze M, Antelmi A, Hamnerius N, Hauksson I, Ulriksdotter J, et al. Continuous glucose monitoring systems give contact dermatitis in children and adults despite efforts of providing less ‘allergy- prone’ devices: investigation and advice hampered by insufficient material for optimized patch test investigations. J Eur Acad Dermatol Venereol 2021; 35: 730–737.
- Mowitz M, Herman A, Baeck M, Isaksson M, Antelmi A, Hamnerius N, et al. N,N-dimethylacrylamide-A new sensitizer in the FreeStyle Libre glucose sensor. Contact Dermatitis 2019; 81: 27–31.
- Ulriksdotter J, Mowitz M, Svedman C, Bruze M. Patch testing and diagnosis when suspecting allergic contact dermatitis from medical devices. Contact Dermatitis 2020; 83: 333–335.
- Johansen JD, Aalto-Korte K, Agner T, Andersen KE, Bircher A, Bruze M, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing – recommendations on best practice. Contact Dermatitis 2015; 73: 195–221.
- Fregert S. Manual of contact dermatitis. Copenhagen: Munksgaard; 1981.
- Diepgen TL, Ofenloch RF, Bruze M, Bertuccio P, Cazzaniga S, Coenraads PJ, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol 2016; 174: 319–329.
- Rossi M, Coenraads PJ, Diepgen T, Svensson Å, Elsner P, Gonçalo M, et al. Design and feasibility of an international study assessing the prevalence of contact allergy to fragrances in the general population: the European Dermato-Epidemiology Network Fragrance Study. Dermatology 2010; 221: 267–275.
- Naldi L, Cazzaniga S, Gonçalo M, Diepgen T, Bruze M, Elsner P, et al. Prevalence of self-reported skin complaints and avoidance of common daily life consumer products in selected European Regions. JAMA Dermatol 2014; 150: 154–163.
- de Groot AC. Myroxylon pereirae resin (balsam of Peru) – a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis 2019; 80: 335–353.
- Dendooven E, Foubert K, Naessens T, Pieters L, Lambert J, Goossens A, et al. Allergic contact dermatitis from (“hypoallergenic”) adhesives containing D-limonene. Contact Dermatitis 2022; 86: 113–119.
- Heine G, Schnuch A, Uter W, Worm M. Type-IV sensitization profile of individuals with atopic eczema: results from the Information Network of Departments of Dermatology (IVDK) and the German Contact Dermatitis Research Group (DKG). Allergy 2006; 61: 611–616.
- Hamann CR, Hamann D, Egeberg A, Johansen JD, Silverberg J, Thyssen JP. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis. J Am Acad Dermatol 2017; 77: 70–78.
- Paulsen E, Andersen KE. Sensitization patterns in Compositae-allergic patients with current or past atopic dermatitis. Contact Dermatitis 2013; 68: 277–285.
- Herman A, Baeck M. Sensitization to isobornyl acrylate in a tertiary Belgian hospital. Contact Dermatitis 2021; 85: 105–106.
- Christoffers WA, Coenraads PJ, Schuttelaar ML. Two decades of occupational (meth)acrylate patch test results and focus on isobornyl acrylate. Contact Dermatitis 2013; 69: 86–92.
- Dendooven E, Dirinck E, Foubert K, Aerts O. “Re-testing” suggests that cosensitizations to isobornyl acrylate and sesquiterpene lactones may be due to cross-reactivity. Contact Dermatitis 2022; 86: 57–59.
- Oppel E, Kamann S, Reichl FX, Hogg C. The Dexcom glucose monitoring system – an isobornyl acrylate-free alternative for diabetic patients. Contact Dermatitis 2019; 81: 32–36.
- Oppel E, Kamann S, Heinemann L, Klein A, Reichl FX, Högg C. Freestyle libre 2: the new isobornyl acrylate free generation. Contact Dermatitis 2020; 83: 429–431.
- Peeters C, Herman A, Goossens A, Bruze M, Mowitz M, Baeck M. Allergic contact dermatitis caused by 2-ethyl cyanoacrylate contained in glucose sensor sets in two diabetic adults. Contact Dermatitis 2017; 77: 426–429.