Psoriatic patients with latent tuberculosis infection and properly treated active tuberculosis need careful management when prescribing modern biological drugs. Although data and guidelines regarding tumour necrosis factor-α inhibitors advise caution and initiation of prophylactic therapy in patients with latent tuberculosis infection, the same indications do not seem to find equal force for interleukin (IL)-23 and IL-17 inhibitors. In order to evaluate the risk of reactivation in patients with latent tuberculosis infection or properly treated active tuberculosis, an observational retrospective study was conducted on the population referred to our centre at Dermatologic Clinic of University of Turin, Italy. In the last 10 years at the clinic 19 psoriatic patients were found to be at risk of tuberculosis reactivation: 10 patients were QuantiFERON- TB-positive at baseline, 2 became positive during treatment, 6 reported prior tuberculous infection, and 1 was QuantiFERON-TB-negative at baseline and developed disseminated tuberculosis during treatment with anti-tumour necrosis factor-α. Overall, 10.5% of this group of patients developed active tuberculosis; however, stratifying by biologic therapy, zero cases were observed among patients treated with anti-IL-17, -23, or -12/23 over a relatively long follow-up (48.1 months) A review of the available literature following our experience confirms the increased risk of tuberculosis reactivation with tumour necrosis factor-α inhibitors. Concerning anti-IL-23 and IL-17 drugs, available data showed high safety in patients at risk of tuberculosis reactivation. Screening of patients who should be taking IL-17 and IL-23 inhibitors is recommended for public health purposes. In case of a positive result with these therapies, consulting with an infectious diseases specialist is suggested in order to weigh up the risks and benefits of prophylactic treatment.
Key words: tuberculosis; infection; psoriasis; biologics.
Accepted Sep 6, 2022; Epub ahead of print Sep 6, 2022
Acta Derm Venereol 2022; 102: adv00821.
DOI: 10.2340/actadv.v102.1982
Corr: Simone Ribero, Dermatology Clinic, Department of Medical Sciences, University of Turin, via Cherasco 23, IT-10121 Turin, Italy. E-mail: simone.ribero@unito.it
SIGNIFICANCE
Patients with a history of latent tuberculosis infection may be at increased risk of reactivation while on biologic therapies. Tuberculosis-related events occurred in 4 of 19 patients in our cohort, all of whom were being treated with tumour necrosis factor-α inhibitors. We recommend initial screening with a QuantiFERON-TB test, use of anti-interleukin-23 and -17 drugs, and possible consultation with an infectious disease specialist for this population.
INTRODUCTION
Psoriasis is a chronic systemic inflammatory disease that predominantly affects the skin and joints (1). Disturbances in the innate and adaptive cutaneous immune responses are responsible for the development and sustainment of psoriatic inflammation. The inflammatory pathways involved include tumour necrosis factor (TNF)-α, interferon (IFN)-α and -β, and interleukins (ILs) -17, -12 and -23 (2). Mild and moderate forms of psoriasis may benefit from topical therapy based on steroids plus vitamin D derivatives in the first line, while, in case of poor response or increased severity, the use of systemic therapies may be considered (methotrexate, cyclosporine, dimethyl fumarate, etc.) (3, 4). Over the past 20 years, therapy with biologics has resulted in significant responses, but immune depression remains a challenge (5).
Previous contact with Mycobacterium tuberculosis (Mtb) exposes patients treated with biologics to an increased risk of tuberculosis (TB) reactivation. Tests for latent tuberculosis infection (LTBI) are used for identifying patients with higher odds of TB reactivation, and treatment is suggested in candidates undergoing immune suppressive treatments (6).
The interpretation of either interferon-gamma release assays or tuberculin skin tests, and the sensitivity of these screening tests, is controversial (7, 8). The increased use in clinical practice of biologics in recent years and the associated risk of LTBI reactivation raise questions about the need for routine screening tests, and the possible subsequent management of LTBI patients with psoriasis (9).
Targeting different pathways, the inhibitors of TNF-α (adalimumab, infliximab, etanercept and certolizumab-pegal), of IL-12/IL-23 (ustekinumab), of IL-17 (secukinumab, ixekizumab, brodalumab) and of IL-23 (risankizumab, tildrakizumab, guselkumab) have a differential impact on risk of LTBI reactivation (7). Clinical practice and the screening test for biologics in psoriasis was originally based on adalimumab, and then applied to the other anti-ILs, but currently it seems more appropriate to differentiate this approach according to the effective drug-associated risk.
This paper summarizes the most recent immunological and clinical evidence on the role of biologics and their safety with regards to Mtb infection, including the clinical experience of our tertiary referral centre, the Dermatology Clinic of the University of Turin, Turin, Italy.
METHODS
An observational retrospective study was performed. Inclusion criteria were: all adult patients who underwent treatment with any approved biologics for moderate-to-severe psoriasis from January 2010 to November 2021 at the Dermatology Clinic of Turin University Hospital; patients presenting a positive screening QuantiFERON-TB (QTF) Gold (DiaSorin, Saluggia, Italy and QUIAGEN®, Hilden, Germany) test with no evidence of active tuberculosis (ATB) (negative chest X-ray, no compatible signs and symptoms); patients considered at high risk of reactivation and labelled as LTBI; or a prior history of properly treated active tuberculosis (pATB) with or without a positive screening QTF, or a negative pre-biologics QTF with subsequent development of a positive QTF or ATB under treatment.
Demographic and clinical data were recorded. The study was approved by the ethics committee of Turin University hospital (IT10771180014 SS-Dermo20).
RESULTS
A total of 540 patients with moderate-to-severe psoriasis were followed in our dermatologic clinic of the University of Turin from November 2011 to November 2021. Of these, 19 patients met the inclusion criteria. The patients’ characteristics are summarized in Table I.
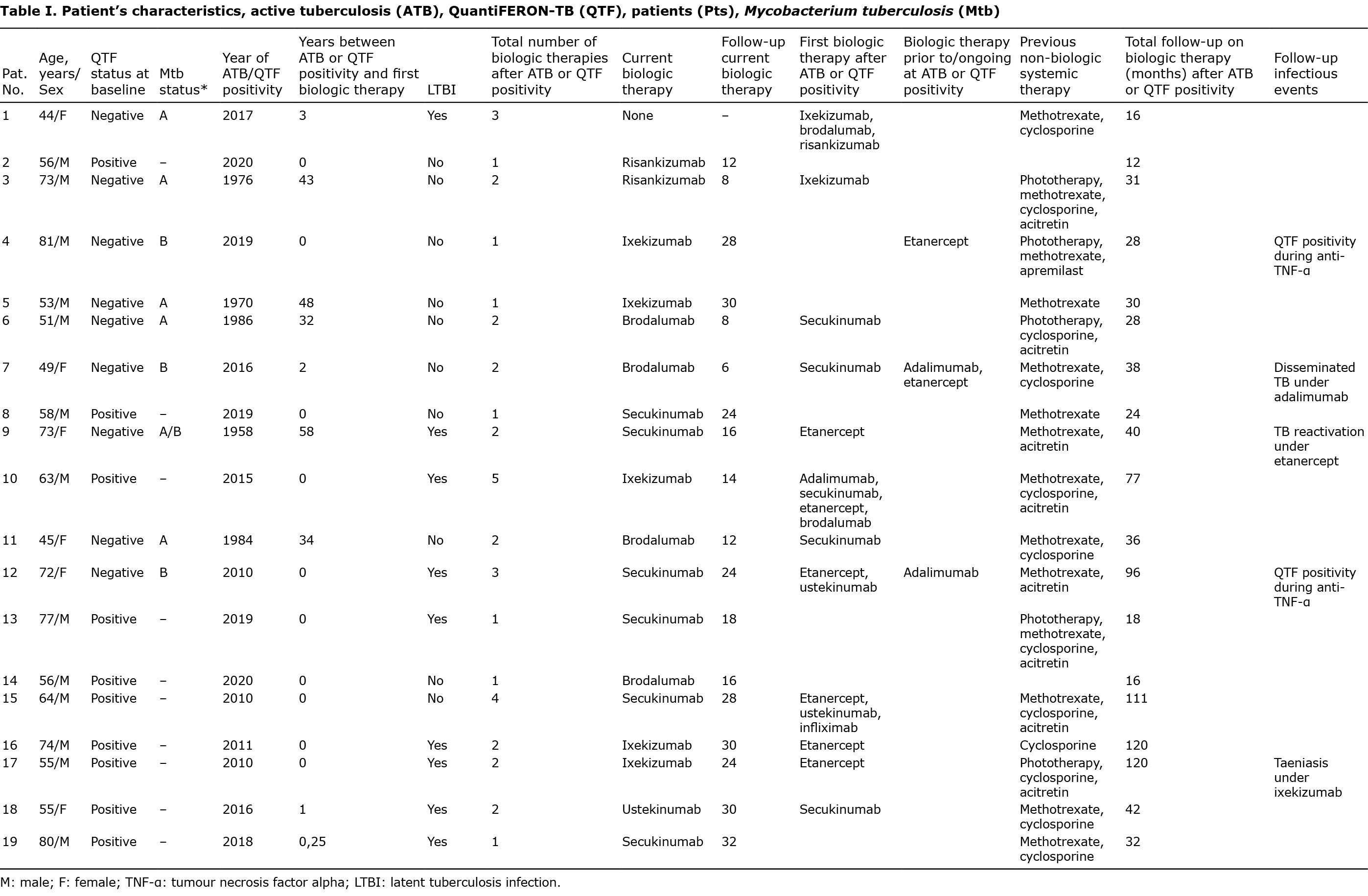
Six patients had pATB: 5 developed TB during their youth, and 1 patient developed TB 2 years before the first biologic therapy, during the screening for systemic therapy for psoriasis. The mean time from pATB to the start of the first biologics was 36.3 years (range 3–58 years). According to the clinical records all these patients had negative QTF at first biologic prescription.
One case of TB reactivation occurred in the pATB group. A male patient who experienced pATB 58 years previously developed TB reactivation after 24 months on etanercept, despite commencing isoniazid prophylaxis 1 month prior to starting treatment with biologics. He stopped etanercept, underwent appropriate anti-tubercular treatment and was switched to secukinumab with complete response of his psoriasis after 16 months without any further reactivation.
One female subject with baseline-negative QTF developed disseminated TB after 2 months of adalimumab and 10 months of etanercept.
Ten patients had a positive QTF at the baseline screening for the first biologic prescription (10 subjects). Among them, biologics were started after a mean time of 1.5 months, 8 patients started the treatment immediately after positive QTF, 1 patient after 3 months, and 1 after 12 months from the positive result. No patients in the positive QTF group experienced TB reactivation.
Two patients had positive QTF during follow-up while under anti-TNF-α treatment (1 patient on adalimumab and 1 on etanercept) after a negative baseline.
Overall, 9 patients underwent treatment for LTBI before administration of biologics: 7 in the QTF group and 2 in ATB. As for the latter, these 2 patients were deemed at high risk of reactivation despite previous treatment, as they were not able to precisely recall drugs and duration of previous anti-tubercular treatment, and after infectious disease consultation they underwent prophylaxis. Nine of the 10 patients without prophylactic treatment received only anti-IL17 or anti-IL-23 biological treatments, while 1 patient with positive QTF did not receive prophylaxis, due to concomitant severe liver disease. Infectious disease consultation deemed hepatotoxicity to overcome the risk of LTBI reactivation despite the indication to start infliximab and etanercept; no LTBI reactivation was observed during and after the overall 24 months under TNF-α.
The mean follow-up time under psoriatic biologic therapy was 48.1 months (range 12–120). Sixteen patients had a regular follow-up. One patient is not currently taking biological therapy for psoriasis due to multiple failures after brodalumab, ixekizumab, and risankizumab. Eighteen patients are currently on biologics, 6 of whom are on secukinumab, 4 on brodalumab, 5 on ixekizumab, 2 on risankizumab, and 1 on ustekinumab. The mean follow-up duration under each biologic is reported in Table II.

Overall, 2/19 (10.5%) cases of ATB were observed in this group of patients at increased risk of LTBI reactivation based on previous ATB history or positive screening; nevertheless, when stratified by biologics, zero cases were observed among patients treated with anti-IL-17, -IL-13, or -IL-12/23 on a relatively long follow-up.
DISCUSSION
Biology of latent tuberculosis infection
LTBI should be considered as a dynamic spectrum of conditions that depend on mycobacteria burden and replication as well as on hosts’ inflammatory status and immune system efficacy (10, 11). Reactivation may even occur asymptomatically, and spontaneously return to a deeper pathologically latency. Mechanisms that allow progression from latency to clinical manifestations are unclear (10, 11).
Granulomas represent the immunological and mechanical barrier to control Mtb. Maintenance of granulomas is a dynamic process involving changes in CD4+ and CD8+ T-cells, B-cells, macrophages, neutrophils, fibroblasts, and multinucleated giant cells (11). Several cytokines, including TNF-α, IL-12/IL-23 and IL-17 are involved (Table SI). Adaptive immunity to Mtb depends mainly on CD4+ T-cells, the major producers of IFN-γ, contributors of TNF-α production, and regulators of optimal CD8+ T-cells functioning (11, 12).
Anti-TNF-α therapy can have a negative impact on the long-lasting maintenance of granuloma integrity, on TNF-α-induced macrophages apoptosis, and on the enhanced intracellular killing of Mtb (12–14).
The IL-12 family (including IL-12 and IL-23) and IL-17 pathway are involved during early protective responses and in boosting vaccine-induced immunity,
In early infections, IL-12p70 (IL-12p35/p40) triggers dendritic cells, promotes macrophage migration, induces IFN-γ production from CD4+ and NK cells, and the differentiation of Th1 effectors (15). IL-23 (IL-23p40/p19) induces the differentiation of Th17 cells, associated with MTb protection during primary infection, and plays a secondary role to IL-12 in inducing IFN-γ-mediated responses (16), genetic defects in IL-12/IL-23/IFN-γ axis have been associated with severe and disseminated disease (17), IL-12/IL-23- deficient mice have been found to be more susceptible to Mtb than IL-12-deficient mice (18), and IL-23- deficient mice have shown normal protective immunity and mycobacterial burden (15, 19).
IL-17 is an inflammatory cytokine capable of inducing chemokine gradients and initiating inflammation, especially in mucosal tissues (16). While IL-17 seems beneficial during primary Mtb infection, it may become detrimental during chronic infection (11, 13, 15, 16). IL-17 appears to favour the control of tuberculous granuloma in human models by increasing specific tissue-resident memory T-cells (CD4+ and CD8+) (20).
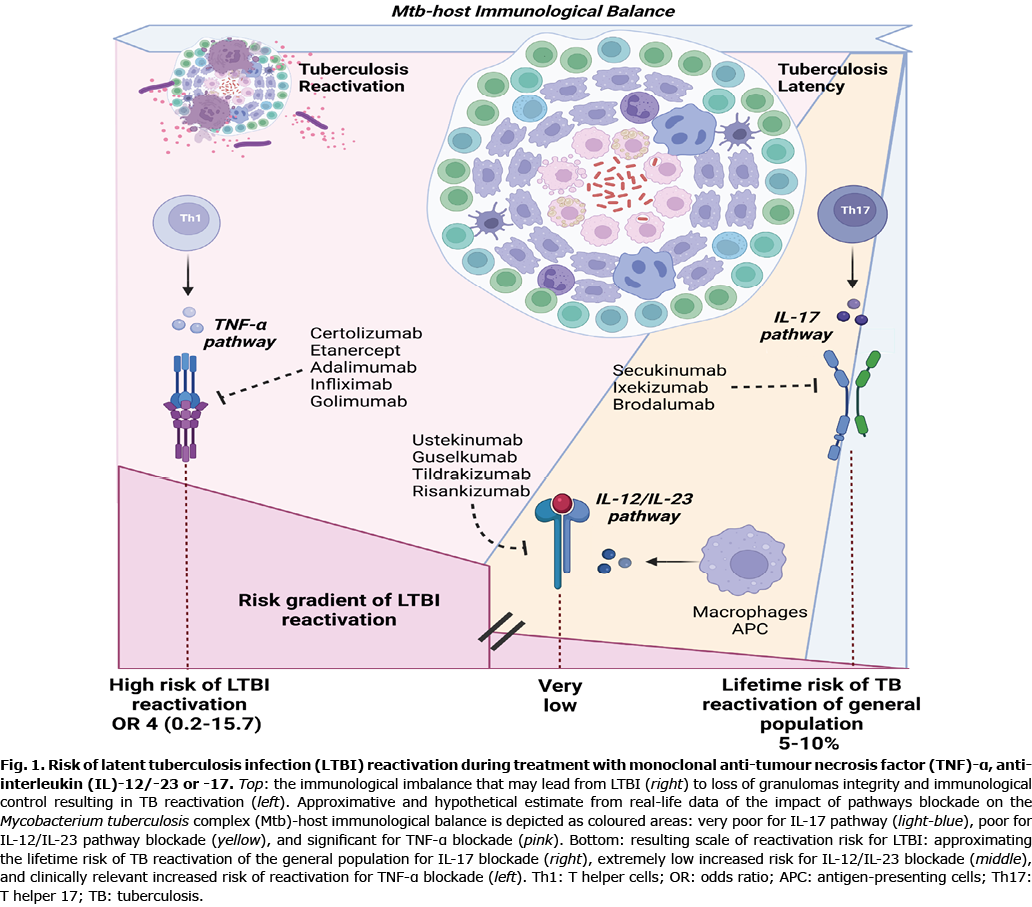
A schematic representation of the comparison in LTBI reactivation risk between TNF-α, IL-12/IL-23, and IL-17 pathway blockades is shown in Fig. 1.
Literature review of clinical data
Interpretation of the currently available data is difficult due to the differentiation in the various studies between tuberculous reactivation, new tuberculous infection, and positive tests, such as tuberculin skin test (TST) and IFN-gamma releasing essay (IGRA, i.e. QuantiferonTB) after negative baseline screening. The different epidemiological risk within the European area and the variable sensitivity of current screenings, and their interpretation, further complicate the risk assessment of TB reactivation in psoriatic patients treated with biologics (9). The percentage of psoriatic patients with LTBI in Europe is approximately 20%, while in Italy this incidence has been reported to be lower (8.2%) (21).
Recent European guidelines recommend Mtb screening according to local regulations (7). The screening should be based on the patient’s personal history, physical examination, chest X-ray, and laboratory tests, such as TST and IGRA; the guidelines also recommend repeated screening during treatment. Different types of prophylaxis are available for LTBI: isoniazid for 6 months, isoniazid+rifampin for 3 months, or rifampin alone for 3-4 months, all associated with an 85–95% reduction in the risk of TB reactivation (7, 22).
With methotrexate, an increased risk of tubercular reactivation is evidenced (23). The frequent use of this treatment before initiation of biological therapy may cause confusion regarding which drugs cause LTBI reactivation. Of the patients in this study, 73.6% were treated with methotrexate before biologics, and both cases that developed ATB belonged to this group.
Tumour necrosis factor-α inhibitors
The European guidelines advise against the use of anti-TNF-α in patients with LTBI. In this type of patient, systemic treatment with retinoids and dimethyl fumarate, and biological drugs based on IL-17 and IL-23, are suggested (7).
The risk of TB reactivation in psoriatic patients treated with anti-TNF-α is ascertained from several clinical trials and real-world evidence, in particular adalimumab and infliximab (and less strongly for etanercept) (24). A review conducted in 2013 on 13 clinical trials conducted from 2003 to 2012 on psoriatic patients on anti-TNF-α showed 6 cases of TB reactivation out of 3,657 total patients, 1 case with adalimumab and 5 with infliximab (5). Souto et al. highlighted an odds ratio of 1.91, with 31 case of TB under TNF inhibitors for immune-mediated disease (25). Minozzi et al. conducted a systematic review encompassing data from 71 published randomized controlled trials (RCTs) involving 22,760 adult patients with a rheumatological disease treated with anti-TNF-α, and from 7 open label extension studies with 2,236 patients highlighting an increase in the occurrence of TB (250%) associated with anti-TNF drug use (26). ATB was recently reported in a patient receiving certolizumab-pegol treatment (27, 28).
Tuberculous screening before starting biological treatment does not always correctly identify patients who are at risk of reactivation (8). Numerous cases, even fatal cases, of tuberculous reactivation in patients with negative screening, are reported in the literature (29). Similarly, positive QTF results after negative baseline during treatment with biologics appear to be difficult to evaluate. A retrospective study reported positive QTF of 6.5% after negative baseline screening in 526 patients treated with anti-TNF, anti-IL-17, and anti-IL-12/23 (21, 30). In the current population 2 patients experienced QTF conversion during treatment with anti-TNF-α and ustekinumab. A female patient from the current population developed disseminated TB with involvement of the brain, liver, and bones, after negative QTF screening and treatment with etanercept and adalimumab; in this case the clinical history strongly oriented towards a case of reactivation rather than a primary Mtb infection. Recent evidence confirms low risk of reactivation in LTBI patients under anti-TNF-α treatment for psoriasis after prophylactic treatment (31, 32). Nevertheless, cases of LTBI reactivation in patients who received prophylaxis have also been described (33, 34). In the current series, a patient who had ATB almost 60 years earlier, and despite adequate prophylaxis, experienced reactivation of pulmonary TB after 2 years of etanercept-based therapy.
Interleukin-12/interleukin-23 inhibitors
Ustekinumab is not traditionally considered to increase the risk of tuberculous reactivation, although preclinical studies attribute a major role to IL-12, and to a lesser extent IL-23, in controlling TB infection (35). A recent Korean study showed an incidence of TB in the population treated with ustekinumab comparable with that of the general population (35). To our knowledge, 10 cases of tuberculous reactivation after therapy with ustekinumab are reported in the literature, and of similar size is the population that experienced QTF conversion during the treatment (21, 25, 36–39). In our series, 3 patients were treated with ustekinumab for a median follow-up of 28 months; 2 underwent prophylactic treatment, and no case of LTBI reactivation occurred.
Interleukin-17 inhibitors
Regarding secukinumab, out of 7,355 patients in phase I, II, III trials, 132 patients had LTBI or reported pATB, 107 underwent prophylactic therapy, and 25 patients with pATB that resulted negative to screening received secukinumab without anti-TB treatment. No case of reactivation was observed (40). An Italian real-life study on 12 patients with LTBI showed no reactivation on secukinumab without previous prophylactic treatment after a 52-week follow-up (6). Further real-life studies have reported regular follow-up in LTBI patients treated with secukinumab without prophylactic therapy, despite 10 cases of QTF positivity during treatment (21, 41, 42). In the current series, 11 patients have taken secukinumab, and 6 are still currently on it. Four patients did not receive prophylactic treatments, 2 of these had negative screening, but pATB, while 1 received anti-tubercular treatment before starting secukinumab due to disseminated TB. Overall, the mean follow-up was 23.6 months with no cases of LTBI reactivation. In line with previous results, the current data do not suggest an increased risk of developing TB or reactivation in patients treated with secukinumab, despite significant exposure to Mtb.
Unlike secukinumab, the phase I, II, and III studies of ixekizumab and brodalumab excluded LTBI-positive patients at screening. Regarding ixekizumab, out of 7,016 patients being treated for psoriasis, 133 experienced emergent LTBI, 48 remained on treatment, 11 did not receive anti-TB treatment, and 9 discontinued treatment. No cases of reactivation were reported at five-year follow-up data in UNCOVER-1 and -2 studies in 206 patients (43).
Regarding brodalumab, in 4,464 patients in phase I, II, III studies, no cases of reactivation were reported. An extension study on 129 patients at 108 weeks also reported no cases of reactivation (44).
The current series is the first to report real-life data on ixekizumab and brodalumab in psoriatic patients at risk of tuberculous reactivation. Seven patients were treated with ixekizumab for a mean follow-up of 21.6 months without reactivation. Three out of 7 patients did not receive prophylactic treatment, 2 due to pATB at a young age with negative screening, and 1 patient because ixekizumab was not deemed to be associated with risk of reactivation.
Six patients received brodalumab for a mean follow-up of 10.3 months without reactivation.
Interleukin-23 inhibitors
In phase II and III trials on IL-23-inhibitors, 105 psoriatic patients with LTBI on guselkumab did not report reactivation (40).
From the phase I, II, and III studies of risankizumab for psoriasis, 72 patients tested positive for LTBI and received prophylactic therapy, with no cases of reactivation (40). Thirty-one patients who tested positive at QuantiFERON-TB in the IMMhance study did not receive prophylactic therapy without reactivation cases at 55 weeks follow-up. Overall, no cases of reactivation were reported in studies involving 2,673 treated patients (40, 45).
Two patients with pATB and 1 with positive QTF received risankizumab in our clinic. Only 1 patient with pATB underwent prophylaxis; in consideration of the absence of cases of reactivations in the literature, prophylaxis was not given in the other patients. After a mean of 9.3 months of follow-up, no cases of tuberculous reactivation occurred. To date, the current data are the only real-life experience of risankizumab in patients at risk of LTBI reactivation.
Final considerations
Concerning the anti IL-23 and IL-17 drugs, the data in the literature show high safety in patients with LTBI and previous ATB, and data are also available from patients at risk who did not receive prophylactic therapy without proof of reactivation. Seven cases of de novo ATB, thus considered unrelated to treatments, are reported in these classes (5 secukinumab, 1 tildrakizumab, and 1 ixekizumab) (40). In the current study population, irrespective of a positive history of LTBI and ATB and possible prophylactic therapy, no cases of reactivation occurred during treatment with brodalumab, secukinumab, ixekizumab, and risankizumab.
In the light of this emerging evidence, after an initial risk assessment using QTF, in the event of a positive result, anti-IL-23 or anti-IL-17 drugs are prescribed without starting prophylactic therapy, but patients are still referred and linked to infectious disease or pneumologist care and consultation. Indeed, as for other autoimmune disorders (46), there is controversial evidence (47–50) and plausible biological explanations (51, 52) of an increased risk of TB even in biologic-naïve patients with psoriasis., we do not endorse an abrupt cessation of TB screening before and during any type of biologics in psoriatic patients. Rather, we suggest preserving this strategy from a broader public health point of view, waiting for cost-effectiveness analysis and an in-depth assessment of TB vs prophylaxis risks in biologic-naïve patients, as well as in the trajectory of primary Mtb infections during blockade of IL-12, IL-23 and IL-17 pathways. Indeed, if a first line has to be started with these inhibitors, the physician may not wait for TB screening results before administering the treatment. In case of a positive screening, an infectious disease consultation will balance the risks and benefits and the urgency of eventual anti-tubercular prophylaxis.
Conclusion
Despite in-depth experience with biologics in the treatment of psoriasis, there are still several controversial data on the assessment and quantification of the risk of tuberculous reactivation. The current data fit with the emerging evidence on the safety of IL-12/IL-23, IL-23 and IL-17 inhibitors in terms of LTBI reactivation, and add preliminary evidence for newer drugs with as-yet no observational data. While these findings endorse the idea that Mtb screening may be no longer mandatory before starting anti-IL-12/IL-23, -IL-23 or -IL-17 therapy, important caveats remain to be discussed and a consensus needs to be reached on the usefulness and interpretation of Mtb screening in this population of patients.
ACKNOWLEDGEMENTS
The study was approved by the ethics committee of Turin University Hospital (IT10771180014 SS- Dermo20), and was conducted according to the principles of the Declaration of Helsinki.
Informed consent was obtained from all subjects involved in the study.
Data are available from the corresponding author upon reasonable request.
The authors have no conflicts of interest to declare.
REFERENCES
- Dapavo P, Siliquini N, Mastorino L, Avallone G, Merli M, Agostini A, et al. Efficacy, safety, and drug survival of IL-23, IL-17, and TNF-alpha inhibitors for psoriasis treatment: a retrospective study. J Dermatolog Treat 2022; 33: 2352–2357.
- Cariti C, Dapavo P, Mastorino L, Ortoncelli M, Siliquini N, Merli M, et al. Comparison of secukinumab and ixekizumab in psoriasis: a real-life cohort study on the efficacy and drug survival of 445 patients. J Eur Acad Dermatol Venereol 2022; 36: e233–e235.
- Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers 2016; 2: 16082.
- Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris – part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol 2020; 34: 2461–2498.
- Solovan C, Chiticariu E. Psoriasis, anti-tumor necrosis factor therapy, and tuberculosis: report of three challenging cases and literature review. Infect Dis Ther 2013; 2: 59–73.
- Ribero S, Licciardello M, Quaglino P, Dapavo P. Efficacy and safety of secukinumab in patients with plaque psoriasis and latent tuberculosis. Case Rep Dermatol 2019; 11: 23–28.
- Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris – Part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol 2021; 35: 281–317.
- Bassukas ID, Gaitanis G, Constantopoulos SH. Diagnosis of tuberculosis in patients with psoriasis: the need for a modified approach. Eur Respir J 2011; 38: 231–232; author reply 232–233.
- Ahn CS, Dothard EH, Garner ML, Feldman SR, Huang WW. To test or not to test? An updated evidence-based assessment of the value of screening and monitoring tests when using systemic biologic agents to treat psoriasis and psoriatic arthritis. J Am Acad Dermatol 2015; 73: 420–428.e1.
- Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009; 7: 845–855.
- Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol 2012; 12: 581–591.
- Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol 2010; 185: 15–22.
- Sia JK, Rengarajan J. Immunology of mycobacterium tuberculosis infections. Microbiol Spectr 2019; 7: 10.1128/microbiolspec.GPP3-0022-2018.
- Mohan VP, Scanga CA, Yu K, Scott HM, Tanaka KE, Tsang E, et al. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect Immun 2001; 69: 1847–1855.
- Méndez-Samperio, P. Role of interleukin-12 family cytokines in the cellular response to mycobacterial disease. Int J Infect Dis 2010; 14: e366–371.
- Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine 2008; 41: 79–83.
- Ottenhoff THM, Verreck FAW, Lichtenauer-Kaligis EGR, Hoeve MA, Sanal O, van Dissel JT. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet 2002; 32: 97–105.
- Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 P40 subunit is present. J Immunol 2002; 168: 1322–1327.
- Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 Compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses If IL-12p70 is available. J Immunol 2005; 175: 788–795.
- Ogongo P, Tezera LB, Ardain A, Nhamoyebonde S, Ramsuran D, Singh A, et al. Tissue-resident-like CD4+ T cells secreting IL-17 control mycobacterium tuberculosis in the human lung. J Clin Invest 2021; 131: e142014.
- Megna M, Ferrillo M, Ruggiero A, Cinelli E, Gallo L, Fabbrocini G. QuantiFERON TB-gold conversion rate among psoriasis patients under biologics: a 9-year retrospective study. Int J Dermatol 2021; 60: 352–357.
- Gisondi P, Pezzolo E, Lo Cascio G, Girolomoni G. Latent tuberculosis infection in patients with chronic plaque psoriasis who are candidates for biological therapy. Br J Dermatol 2014; 171: 884–890.
- Ting SW, Ting SY, Lin YS, Lin MS, Kuo G. Association between different systemic therapies and the risk of tuberculosis in psoriasis patients: a population-based study. Int J Clin Pract 2021; 12: e15006.
- Hernandez C, Cetner AS, Jordan JE, Puangsuvan SN, Robinson JK. Tuberculosis in the age of biologic therapy. J Am Acad Dermatol 2008; 59: 363–380; quiz 382–384.
- Souto A, Maneiro JR, Salgado E, Carmona L, Gomez-Reino JJ. Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatology (Oxford) 2014; 53: 1872–1885.
- Minozzi S, Bonovas S, Lytras T, Pecoraro V, González-Lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 2016; 15: 11–34.
- Snast I, Bercovici E, Solomon-Cohen E, Avni T, Shitenberg D, Hodak E, et al. Active tuberculosis in patients with psoriasis receiving biologic therapy: a systematic review. Am J Clin Dermatol 2019; 20: 483–491.
- Lau CS, Chen YH, Lim K, de Longueville M, Arendt C, Winthrop K. Tuberculosis and viral hepatitis in patients treated with certolizumab pegol in Asia-Pacific countries and worldwide: real-world and clinical trial data. Clin Rheumatol 2021; 40: 867–875.
- Dantes E, Tofolean DE, Fildan AP, Craciun L, Dumea E, Tofolean IT, et al. Lethal disseminated tuberculosis in patients under biological treatment – two clinical cases and a short review. J Int Med Res 2018; 46: 2961–2969.
- Megna M, Ruggiero A, Ferrillo M, Fabbrocini G. QuantiFERON-TB Gold conversion is not uncommon in patients with psoriasis undergoing anti-tumour necrosis factor-α therapy. Br J Dermatol 2020; 183: 977–978.
- Narcisi A, Bernardini N, Orsini D, D’Agostino M, De Felice C, Di Stefani A, et al. Long-term safety and efficacy of adalimumab in psoriasis: a multicentric study focused on infections (connecting study). Postepy Dermatol Alergol 2020; 37: 428–434.
- Sánchez-Moya AI, Dauden E. Incidence of tuberculosis infection in psoriatic patients on anti-TNF therapy: report of a case series with 144 patients. J Eur Acad Dermatol Venereol 2011; 25: 730–733.
- Oh JH, Ham SP, Park HJ. Disseminated tuberculosis in a psoriasis patient under adalimumab treatment despite the chemoprophylaxis of latent tuberculosis: a case report. Ann Dermatol 2021; 33: 77–81.
- Gori A, Fabroni C, Prignano F, Lotti T. Unusual presentation of tuberculosis in an infliximab-treated patient – which is the correct TB screening before starting a biologic? Dermatol Ther 2010; 23: S1–3.
- Tsai TF, Ho V, Song M, Szapary P, Kato T, Wasfi Y, et al. The safety of ustekinumab treatment in patients with moderate-to-severe psoriasis and latent tuberculosis infection. Br J Dermatol 2012; 167: 1145–1152.
- Lynch M, Roche L, Horgan M, Ahmad K, Hackett C, Ramsay B. Peritoneal tuberculosis in the setting of ustekinumab treatment for psoriasis. JAAD Case Rep 2017; 3: 230–232.
- Errichetti E, Piccirillo A. Latent tuberculosis reactivation in a patient with erythrodermic psoriasis under treatment with ustekinumab and a low dose steroid, despite isoniazid chemoprophylaxis. Eur J Dermatol 2014; 24: 508–509.
- Tsai TF, Chiu HY, Song M, Chan D. A case of latent tuberculosis reactivation in a patient treated with ustekinumab without concomitant isoniazid chemoprophylaxis in the PEARL trial. Br J Dermatol 2013; 168: 444–446.
- Hsiao CY, Chiu HY, Wang TS, Tsai TF. Serial QuantiFERON-TB Gold testing in patients with psoriasis treated with ustekinumab. PLoS One 2017; 12: e0184178.
- Nogueira M, Warren RB, Torres T. Risk of tuberculosis reactivation with interleukin (IL)-17 and IL- 23 inhibitors in psoriasis – time for a paradigm change. J Eur Acad Dermatol Venereol 2021; 35: 824–834.
- Shu D, Zhang Z, Zhou EY, Ma X, Zhao Y. Is chemoprophylaxis necessary for all latent tuberculosis infection patients receiving IL-17 inhibitors? A cohort study. Dermatol Ther 2020; 33: e14512.
- Elewski BE, Baddley JW, Deodhar AA, Magrey M, Rich PA, Soriano ER, et al. Association of secukinumab treatment with tuberculosis reactivation in patients with psoriasis, psoriatic arthritis, or ankylosing spondylitis. JAMA Dermatol 2021; 157: 43–51.
- Leonardi C, Reich K, Foley P, Torii H, Gerdes S, Guenther L, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-severe psoriasis: long-term results from the UNCOVER-1 and UNCOVER-2 phase-3 randomized controlled Trials. Dermatol Ther (Heidelb) 2020; 10: 431–447.
- Yamaguchi Y, Takatsu N, Ootaki K, Nakagawa H. Long-term safety of brodalumab in Japanese patients with plaque psoriasis: an open-label extension study. J Dermatol 2020; 47: 569–577.
- Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156: 649–658.
- Sundbaum JK, Arkema EV, Bruchfeld J, Jonsson J, Askling J, Baecklund E. Tuberculosis in biologic- naïve patients with rheumatoid arthritis: risk factors and tuberculosis characteristics. J Rheumatol 2021; 48: 1243–1250.
- Bassukas ID, Kosmidou M, Gaitanis G, Tsiouri G, Tsianos E. Patients with psoriasis are more likely to be treated for latent tuberculosis infection prior to biologics than patients with inflammatory bowel disease. Acta Derm Venereol 2011; 91: 444–446.
- Balato N, Di Costanzo L, Ayala F, Balato A, Sanduzzi A, Bocchino M. Psoriatic disease and tuberculosis nowadays. Clin Dev Immunol 2012; 2012: 747204.
- Bordignon V, Bultrini S, Prignano G, Sperduti I, Piperno G, Bonifati C, et al. High prevalence of latent tuberculosis infection in autoimmune disorders such as psoriasis and in chronic respiratory diseases, including lung cancer. J Biol Regul Homeost Agents 2011; 25: 213–220.
- Chen YJ, Wu CY, Shen JL, Chen TT, Chang YT. Association between traditional systemic antipsoriatic drugs and tuberculosis risk in patients with psoriasis with or without psoriatic arthritis: results of a nationwide cohort study from Taiwan. J Am Acad Dermatol 2013; 69: 25–33.
- Filoni A, Vestita M, Congedo M, Giudice G, Tafuri S, Bonamonte D. Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels: an observational case-control study. Medicine (Baltimore) 2018; 97: e11185.
- Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin D deficiency and tuberculosis progression. Emerg Infect Dis 2010; 16: 853–855.