In clinical practice, interruption of treatment may not result in immediate cessation of disease control, and some patients even experience sustained treatment response following treatment interruption. This post hoc analysis of UNCOVER-1 and -2 Phase 3 clinical trials characterized the time to loss of treatment response in patients with psoriasis who responded to ixekizumab through a 12-week treatment period, and who were then re-randomized to placebo for the following 48 weeks. For those with static Physician Global Assessment [sPGA]0/1 and Psoriasis Area and Severity Index [PASI]90 at Week 12, the median time to loss of PASI90 was 16.1 weeks (95% confidence interval 12.7–16.4). For those with PASI100 at Week 12, the median time to loss of PASI100 was 12.1 weeks (95% confidence interval 9.0–13.0). A small subset of patients maintained high levels of disease control through Week 60. This study adds to the growing body of evidence on sustained treatment response following treatment interruption.
Key words: ixekizumab; plaque psoriasis; withdrawal; disease modification; remission; sustained off-treatment responses.
Accepted Feb 16, 2022; Epub ahead of print Feb 16, 2022
Acta Derm Venereol 2022; 102: adv00672.
DOI: 10.2340/actadv.v102.1984
Corr: Kim Papp, Probity Medical Research, Inc., Waterloo, Ontario, Canada. E-mail: kapapp@probitymedical.com
SIGNIFICANCE
Some patients continue to experience disease control for a time after treatment stops. To help characterize this phenomenon, we measured the time to loss of disease control in clinical trials, for ixekizumab, a biologic drug used to treat psoriasis. We show that a small number of patients who responded to ixekizumab maintained high levels of disease control throughout the 48 weeks after ixekizumab treatment. We discuss factors which could affect time to loss of treatment response, including how biologics are metabolised and act on the body, natural variation in disease course, removal of disease triggers, and modification of the disease.
INTRODUCTION
Biologics have proven effectiveness and safety in treating plaque psoriasis (PsO). Long-term use of biologics is necessary to control the signs and symptoms of PsO, and it has been demonstrated both in studies with periods of treatment withdrawal and in clinical practice that treatment interruption leads to recurrence of skin signs in patients (1–4). However, sustained responses are observed in a small proportion of patients after treatment withdrawal (1–4). These observations have been interpreted by some as disease modification by biologics (5). The aim of this study was to evaluate sustained treatment responses and time to loss of response in patients receiving ixekizumab (IXE), an anti-interleukin (IL)-17A antagonist, in clinical studies, to complement available data for other biologics used for PsO treatment. This post hoc analysis investigates loss of Psoriasis Area and Severity Index (PASI)75, PASI90 and PASI100 responses, as these are reflective of the more ambitious treatment goals for PsO. In addition, potential explanations for sustained responses are discussed.
MATERIALS and METHODS
The full study protocols, designs, population and outcomes of UNCOVER-1 and -2 have been published previously (6, 7). This post hoc analysis includes only patients who received IXE every 2 weeks (Q2W) through Week (W)12, achieved static Physician Global Assessment (sPGA)0/1 and PASI90 or PASI100 responses at W12, and were re-randomized to placebo (PBO) during the withdrawal period. Efficacy was measured by PASI and sPGA assessments every 4 weeks until W60. The median time to loss of PASI75, PASI90 and PASI100 responses for W12 IXE responders (sPGA0/1 and PASI90 or PASI100) was evaluated using Kaplan–Meier time-to-event analyses. The time to loss of response was calculated as the date of loss of response during the withdrawal period (W16 to W60) minus the date of W12+1. Patients not meeting criteria for loss of PASI response during the withdrawal period were censored at the last visit date during that period and considered non-responders.
RESULTS
Baseline demographics of the overall UNCOVER-1 and -2 population and W12 IXE responders were comparable (Table I). Of the 211 patients who received IXE 80 mg Q2W from baseline and achieved an sPGA0/1 at W12, 180 and 98 patients also had a PASI90 and PASI100 response, respectively, at W12 and were re-randomized to PBO. Our post hoc analysis demonstrated that, for sPGA0/1 and PASI90 responders at W12, the median (95% confidence interval [95% CI]) time to loss of response was 16.1 (12.7–16.4) weeks for PASI90 and 20.1 (17.1–20.6) weeks for PASI75 (Fig. 1A). Of the PASI90 responders at W12, 2.8% (n = 5) did not lose their PASI90 response before W60, while 7.2% (n = 13) did not lose their PASI75 response.
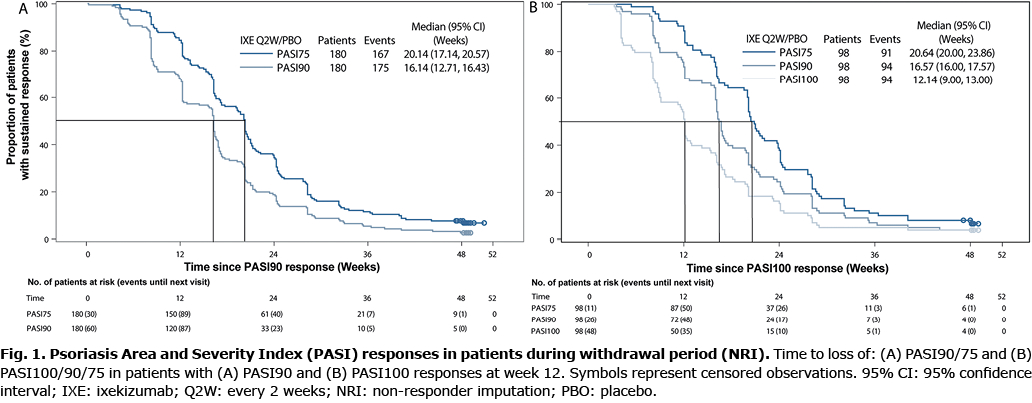
For sPGA0/1 and PASI100 responders at W12, the median (95% CI) time to loss of PASI100 response was 12.1 (9.0–13.0) weeks (Fig. 1B). In addition, the median (95% CI) time to loss of response was 16.6 (16.0–17.6) weeks for PASI90 and 20.6 (20.0–23.9) weeks for PASI75 for the sPGA0/1 and PASI100 responders at W12 (Fig. 1B). Of W12 PASI100 responders, 4.1% (n = 4) did not lose their PASI100 response before W60, 4.1% (n = 4) did not lose their PASI90 response, and 7.1% (n = 7) did not lose their PASI75 response.
DISCUSSION
This post hoc analysis of UNCOVER-1 and -2 shows that, after IXE withdrawal at W12, half of PASI90 and PASI100 responders maintained their response for 16 and 12 weeks, respectively. A small proportion of responders at W12 maintained their response for 48 weeks after IXE withdrawal, consistent with previously published data from UNCOVER-1 and -2, demonstrating that, among IXE-treated patients with an sPGA0/1 response at W12 who were re-randomized to receive PBO, 7.6% maintained an sPGA0/1 response after 48 weeks and had a median time to relapse (defined as an sPGA ≥3) of 20.4 weeks (3, 6).
Sustained off-treatment responses have been reported for some biologics for PsO, including IXE (1–4). The median times to relapse for some biologics for PsO are summarized in Table II; however, differences in the randomized withdrawal period design and relapse definitions prevent inter-drug comparisons. Possible explanations for the duration of time to loss of response and frequency of sustained off-treatment responses for different biologics include pharmacodynamic and/or pharmacokinetic properties of biologics, natural fluctuations in the course of PsO, removal of disease triggers, potential disease modification, or any combination of these factors (5).
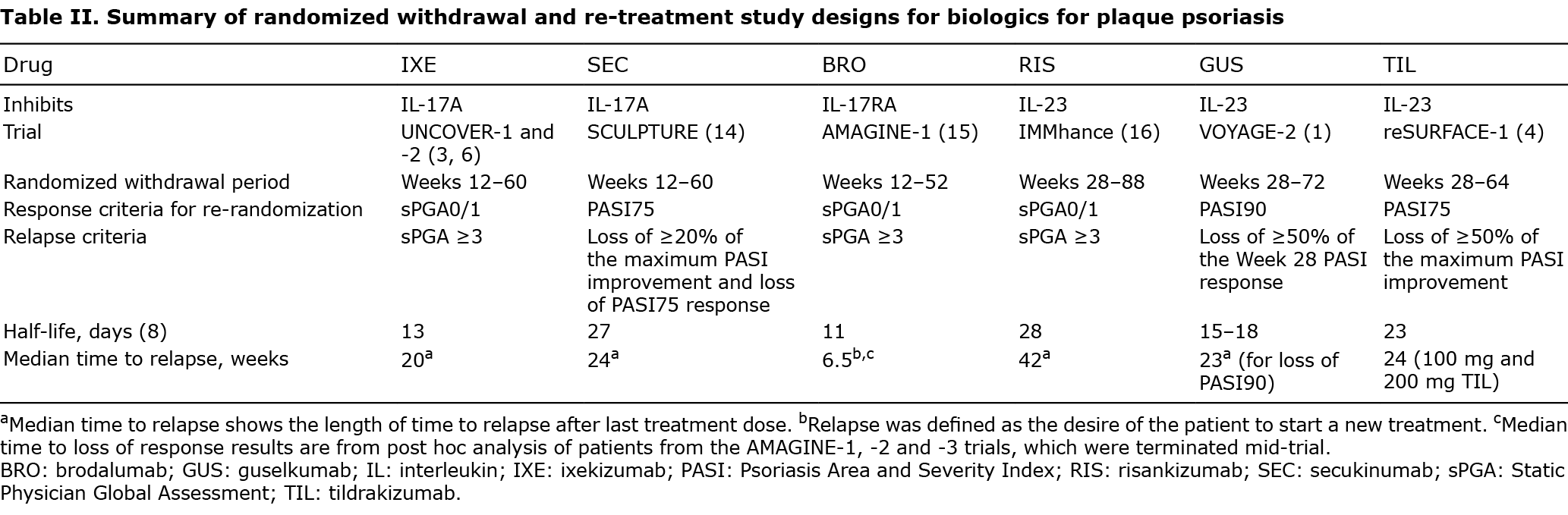
Firstly, variations in pharmacodynamic and/or pharmacokinetic properties between biologics might affect the duration of treatment response. Inhibition of different cytokines involved in PsO may affect distinct inflammatory pathways. In addition, greater proportions of patients achieving high response levels imply a greater proportion with serum drug levels exceeding that required to achieve a response. As the half-lives for anti-IL-17 and anti-IL-23 biologics range from 11 to 28 days (Table II) (8), it may require several months for drug levels to drop below those required to achieve a response threshold, resulting in longer lasting residual effects.
Secondly, PsO is a highly variable disease, ranging from stable disease in some patients to fluctuating periods of relapse and remission in others. Therefore, it is possible that sustained off-treatment responses may be due to a period of natural remission rather than a treatment-specific result. Similarly, removal of disease triggers during treatment periods may reduce disease symptoms during treatment-free periods. Specifically, termination of concurrent medication and lifestyle changes (such as weight reduction, diminished psychosocial stress) may improve PsO symptoms (5). In addition, since being male or biologic-naïve are associated with increased responses to biologic treatment (9), it is possible that these and other factors may also influence off-treatment responses. Conversely, certain genetic variants (such as an IL-23R genotype) and smoking may predispose patients to more severe disease (10), which may potentially accelerate the recurrence of PsO.
Finally, disease modification because of biologic treatment has also been proposed as an explanation for the sustained off-treatment responses observed in some patients. Indeed, in some inflammatory diseases, evidence exists that biologic treatment can modify immunological processes underlying pathogenesis and prevent disease progression (11). While there is no underlying structural, macroscopic damage that can be measured to demonstrate a change in disease progression in PsO, some microscopic changes, such as treatment-recalcitrant tissue-resident memory T cells at sites previously affected by PsO, may potentially exist in PsO; however, further research is needed to investigate this (12).
Reflective of the aforementioned factors, there are no feasible study designs for the investigation of disease modification in PsO. A hypothetical randomized study would need to monitor treatment responders after treatment withdrawal over a defined number of years and compare them with a control group that did not receive any treatment during the initial treatment period. While patients with long-term (often ≥10 years) moderate-to-severe PsO are generally the population investigated in PsO studies, biologic treatment at the early stages of psoriasis is associated with a reduced rate of relapse, and early intensive biologic treatment of other immune-mediated inflammatory diseases suggests this can reduce inflammatory mechanisms that lead to chronic inflammation and improve long-term patient outcomes (5, 11, 13). Therefore, to specifically address the question of disease modification, it appears that patients with new-onset moderate-to-severe PsO should be included in these randomized studies to diminish the impact of the discussed confounders; however, this proposed study design is yet to be validated. In addition, it would be ethically challenging to withhold treatment when highly effective treatments exist for PsO. Overall, as well-designed, adequately powered studies specifically studying disease modification have not been performed in patients with PsO to date, there is insufficient evidence to claim that biologic treatment leads to disease modification in PsO.
In conclusion, this post hoc analysis demonstrated that, for IXE-treated patients who achieved sPGA0/1 and PASI90 or PASI100 at W12, the median time to loss of response was 16 and 12 weeks, respectively, and that a small proportion of patients maintained their PASI responses after 48 weeks. These observations are consistent with IXE’s pharmacokinetic properties and an occasionally fluctuating disease course. Overall, these results and those published for other biologics emphasize a lack of supportive data for disease modification in PsO.
Acknowledgments
The authors thank Gabrielle Stack, a medical writer and employee of Eli Lilly and Company, for writing and editorial support. This study was funded by Eli Lilly and Company, which contributed to study design, data collection, data analysis, data interpretation, manuscript preparation, and publication decisions.
Conflicts of interest: KP has served as a consultant and/or speaker and/or advisor and/or steering committee member and/or scientific officer and/or has received grant/research support/honoraria from: AbbVie, Akros Pharma, Amgen, Anacor Pharmaceuticals, Arcutis, Astellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Bristol Myers Squibb, Can-Fite BioPharma, Celegene, Coherus BioSciences, Dermavent, Dermira, Dice Pharmacueticals, Dow Pharmaceutical Sciences, Eli Lilly and Co., Evelo, Galapagos, Galderma, Genentech, Gilead Sciences, GlaxoSmithKline, Incyte, Janssen, Kyowa Hakko Kirin, LEO Pharma, MedImmune, Meiji Seika Pharma, Merck (MSD), Merck Serono, Mitsubishi Tanabe Pharma, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, Takeda, and UCB Pharma. CP has been a consultant/speaker and an investigator for Almirall, Amgen, AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, LEO Pharma, Merck, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and UCB. EK has received honoraria and/or grants/research funding and/or acted as an advisor/consultant for AbbVie, Almirall, Celgene, Eli Lilly, Janssen, La Roche-Posay, Leo Pharma, Novartis, Pfizer and UCB. Y-HH has conducted clinical trials or received honoraria as a consultant and speaker for AbbVie, Celgene, Janssen-Cilag Pharmaceuticals, Novartis and Pfizer Pharmaceuticals. T-FT is a consultant for and/or is on the speakers’ bureau for: AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly and Co., GSK-Stiefel, Janssen-Cilag, Novartis, and Pfizer. UM has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Aditxt, Almirall, Amgen, Aristea, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Dr Reddy’s, Eli Lilly, Foamix, Formycon, Immunic, Janssen, LEO Pharma, Medac, MetrioPharm, Novartis, Phi-Stone, Pierre Fabre, Sanofi-Aventis, UCB Pharma. CS, AT and ER are employees and minor shareholders of Eli Lilly and Co. CEB is an independent contractor working for Eli Lilly.
REFERENCES
- Reich K, Armstrong AW, Foley P, Song M, Wasfi Y, Randazzo B, et al. Efficacy and safety of guselkumab, an anti- interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418–431.
- Gordon KB, Armstrong AW, Foley P, Song M, Shen YK, Li S, et al. Guselkumab Efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 Study. J Invest Dermatol 2019; 139: 2437–2446.e2431.
- Blauvelt A, Papp KA, Sofen H, Augustin M, Yosipovitch G, Katoh N, et al. Continuous dosing versus interrupted therapy with ixekizumab: an integrated analysis of two phase 3 trials in psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 1004–1013.
- Kimball AB, Papp KA, Reich K, Gooderham M, Li Q, Cichanowitz N, et al. Efficacy and safety of tildrakizumab for plaque psoriasis with continuous dosing, treatment interruption, dose adjustments and switching from etanercept: results from phase III studies. Br J Dermatol 2020; 182: 1359–1368.
- Girolomoni G, Griffiths CE, Krueger J, Nestle FO, Nicolas JF, Prinz JC, et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: a hypothesis paper. J Dermatolog Treat 2015; 26: 103–112.
- Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016; 375: 345–356.
- Griffiths CE, Reich K, Lebwohl M, van de Kerkhof P, Paul C, Menter A, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551.
- Yeung J, Gooderham MJ, Grewal P, Hong CH, Lansang P, Papp KA, et al. Management of plaque psoriasis with biologic therapies in women of child-bearing potential consensus paper. J Cutan Med Surg 2020; 24: 3s–14s.
- Gulliver SR, Gulliver W. Investigation of prevalence of biologic use and discontinuation rates in moderate-to-severe psoriasis patients in Newfoundland and Labrador using real-world data. Dermatol Ther 2021; 34: e14944.
- Svedbom A, Mallbris L, Larsson P, Nikamo P, Wolk K, Kjellman P, et al. Long–term outcomes and prognosis in new-onset psoriasis. JAMA Dermatol 2021; 157: 1–8.
- Allen PB, Olivera P, Emery P, Moulin D, Jouzeau JY, Netter P, et al. Review article: moving towards common therapeutic goals in Crohn’s disease and rheumatoid arthritis. Aliment Pharmacol Ther 2017; 45: 1058–1072.
- Suárez-Fariñas M, Fuentes-Duculan J, Lowes MA, Krueger JG. Resolved psoriasis lesions retain expression of a subset of disease-related genes. J Invest Dermatol 2011; 131: 391–400.
- Huang YW, Tsai TF. Remission duration and long-term outcomes in patients with moderate-to-severe psoriasis treated by biologics or tofacitinib in controlled clinical trials: a 15-year single-center experience. Dermatol Ther (Heidelb) 2019; 9: 553–569.
- Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol 2015; 73: 27–36.e21.
- Galluzzo M, D’Adamio S, Massaro A, Piccolo A, Bianchi L, Talamonti M. Spotlight on brodalumab in the treatment of plaque psoriasis: the evidence to date. Clin Cosmet Investig Dermatol 2019; 12: 311–321.
- Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatology 2020; 156: 649–658.