Elastolytic giant cell granuloma, an idiopathic granulomatous dermatosis, is characterized by annular plaques on sun-exposed areas, and has been termed actinic granuloma or annular elastolytic giant cell granuloma. Many atypical clinical manifestations and lesions involving sun-protected areas have been reported. The aims of this retrospective study of 105 patients were to summarize the clinical and histological features of patients with this condition; to provide evidence for the viewpoint that elastolytic giant cell granuloma is a better term to include all clinical morphological types presenting with elastolysis, elastophagocytosis, and an infiltrate of multinucleated giant cells histologically; and to establish a new clinical classification. The varying clinical manifestations were further categorized into annular, papular, giant, mixed and generalized forms. The pathological manifestations were classified into giant cell, necrobiotic, histiocytic, sarcoidal and mixed patterns. Diabetes mellitus or impaired glucose tolerance were the most commonly identified comorbidities. Oral low-dose corticosteroid may be an effective treatment.
Key words: elastolytic giant cell granuloma; annular elastolytic giant cell granuloma; actinic granuloma; elastolysis; elastophagocytosis.
Accepted Feb 16, 2022; Epub ahead of print Feb 16, 2022
Acta Derm Venereol 2022; 102: adv00684.
DOI: 10.2340/actadv.v102.1985
Corr: Dong-Lai Ma, Department of Dermatology, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, State Key Laboratory of Complex Severe and Rare Diseases, National Clinical Research Center for Dermatologic and Immunologic Diseases, No.1, Shuaifuyuan, Dongcheng District, Beijing 100730, China. E-mail: mdonglai@sohu.com.
SIGNIFICANCE
Elastolytic giant cell granuloma has a wide spectrum of clinical presentations, and misdiagnosis is very common. The term used in the past cannot fully explain the full picture of clinical manifestations. This is the largest series to date to review and summarize the clinical and histological features of patients with elastolytic giant cell granuloma. The current study documented an extensive spectrum of clinical presentations, and suggested that variations in morphologies justify substituting the term elastolytic giant cell granuloma for annular elastolytic giant cell granuloma. In addition, the study established a new clinical classification based on the current findings. Oral low-dose glucocorticoids therapy may be effective against elastolytic giant cell granuloma.
INTRODUCTION
Annular elastolytic giant cell granuloma (AEGCG), an idiopathic type of granulomatous dermatosis, is characterized clinically by annular lesions that expand centrifugally on sun-exposed areas (1). Recently, many atypical variants of this condition, including papular (2), reticular (3), generalized forms (4), concomitant conditions (5) and lesions involving sun-protected areas had been reported. Despite the various clinical presentations, AEGCG is characterized histopathologically by elastolysis, elastophagocytosis, and an infiltrate of multinucleated giant cells. Several comorbidities, including diabetes mellitus (DM) (6), temporal arteritis (7), solid tumours, and haematological malignancy (8) had been reported. Given that lesions of this condition are not always annular and not always in sun-exposed areas, the previous terms “actinic granuloma” (AG) and “AEGCG” seem inappropriate. The terms “giant cell elastolytic granuloma” and “elastolytic giant cell granuloma” (EGCG) have been proposed in the previous studies to define this condition (4, 9).
To determine a better perspective of this disease, a single-centre retrospective study of 105 Chinese patients with EGCG was performed. This is the largest series of patients with EGCG to date. The study aimed to: (i) review and summarize the clinical and histological features in the study cohort; (ii) provide evidence for the viewpoint that EGCG is a better term to include all clinical morphological types presenting with elastolysis, elastophagocytosis, and an infiltrate of multinucleated giant cells histologically; (iii) establish a new clinical classification for EGCG.
MATERIALS AND METHODS
This retrospective hospital-based study was approved by the ethics committee of the authors’ institutions in accordance with the Declaration of Helsinki (ethics number S-K1636). All written patient consents were obtained.
Patients
All patients diagnosed with AG, AEGCG in Department of Dermatology, Peking Union Medical College Hospital between 2002 and 2020 were included. Patients with clinical pictures, complete medical records and formalin-fixed and paraffin-embedded skin biopsy specimens for review were enrolled. The diagnosis of EGCG was re-confirmed by 2 senior dermatologists and 1 dermatopathologist, based on the clinical features, typical histological findings and Verhoeff-Van Gieson staining. Those with granulomatous infiltration and presence of multinucleated giant cells in haematoxylin and eosin (H&E) slides were further stained with Verhoeff-Van Gieson staining. Patients with more than 1 elastophagocytic area in the dermis and presented with phagocytosis of elastic fibres by multinucleated giant cells were considered to be histologically diagnosed. Other infectious or non-infectious granulomatous dermatosis, including sarcoidosis, infectious granulomatous diseases and interstitial granulomatous dermatitis were excluded by histological examinations, fungous microscopic examination, bacteria culture, special staining, and immunological examinations when indicated.
Clinical evaluation
Patients were classified into 5 different clinical subtypes according to their specific morphologies: annular type with predominantly annular erythema; papular type with grouped or scattered papules; giant type with annular lesions with a diameter > 10 cm; mixed type as the co-existence of more than 2 types of clinical manifestations that had similar proportions; and generalized form, with skin involvement > 30% of total body surface area. The distributions on the face, neck, scalp, dorsal sides of the forearms and hands, V-area of the neck, and upper back were defined as sun-exposed areas. The remaining sites were defined as non-exposed areas. The following data were reviewed and summarized in the electronic medical system: sex, age at onset, disease duration, location and distribution of the lesions, clinical manifestations, symptoms and concomitant diseases.
Histological evaluation
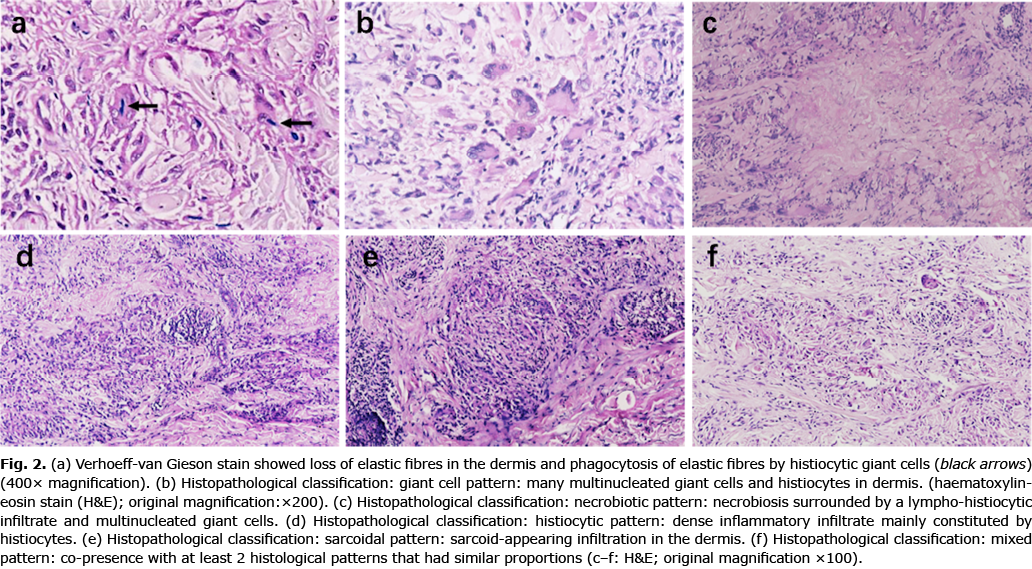
The histopathological features were further classified into 5 patterns: giant cell pattern, with interstitial granulomatous infiltration mainly constituted by multinucleated giant cells in mid-dermis with many broken elastic fibres nearby; necrobiotic pattern, presence of foci of necrobiosis in the upper reticular dermis, surrounded by varying numbers of giant cells; histiocytic variant, consisting of dense inflammatory infiltration mainly constituted by histiocytes and scarce giant cells; sarcoidal pattern, with uniform circumscribed nests of non-caseating granuloma; and mixed pattern, presence with at least 2 histological patterns that had similar proportions.
Statistical analysis
Data are expressed as the mean (standard deviation; SD), number (%) or median (interquartile range; IQR). was used for all statistical analysis. Statistical analysis was performed using the Mann–Whitney U test, Fisher’s precision probability test and Kruskal–Wallis test, where appropriate. A p-value < 0.05 were considered statistically significant. All data were computed using SAS 9.4 (SAS Institute Inc., Kerry, USA) software.
RESULTS
Clinical presentations
The study cohort comprised 105 patients (61 women and 44 men). Mean age at onset was 57.91 ± 12.09 years. At presentation, the mean duration of lesions ranged from 1 month to 21 years. The lesions were located only in sun-exposed areas in 76 patients (72.4%), restricted to sun-protected areas in 7 patients (6.7%), and occurred sporadically in both types of area in 22 patients (20.9%). Their most common location was the dorsum of the hands, followed by the forearms. Other common sites were the face, upper chest and neck. No mucosal or ocular involvement was observed. The lesions were asymptomatic in 80% of patients, the remaining patients reporting varying degrees of pruritus or a burning sensation. Patients were clinically diagnosed as granuloma annulare (GA) (85%), actinic granuloma or annular EGCG, discoid lupus erythema, necrobiosis lipoidica, sarcoidosis, or lichen planus before the histopathological examination.
Classification of elastolytic giant cell granuloma subtype
Five clinical subtypes were identified: annular form; papular form; giant form; mixed form, and generalized form (Table I).

The most common form of EGCG was the annular form, which occurred in 66 patients. The mean age of onset was 56.65 years and the mean disease duration was 2.36 years. Typical annular lesions featured multiple well-demarcated annular or polycyclic erythematous plaques with raised borders and central atrophy and hypopigmentation (Fig. 1a). Some lesions were exacerbated by sun exposure and slowly extended centrifugally. In addition to the typical annular lesions, an extensive spectrum of clinical presentations was documented. Three patients’ lesions had a reticulate appearance that had developed by centrifugal expansion and gradual coalescence of multiple annular plaques (Fig. 1b). Two patients had lesions on the abdomen that had a broad rim of erythema around a lighter centre (Fig. 1c). Scaly and ulcerated borders rather than the smooth borders characteristic of typical annular lesions were noted on lesions on the face and neck in 2 patients (Fig. 1d). Another 2 patients had multiple enlarging, concentric rings that had coalesced and clustered in layers (Fig. 1e). One patient had anatomical co-existing lesions that were typical of annular EGCG and vitiligo (Fig. 1f). This patient’s hypopigmented patches were diagnosed as vitiligo on the basis of findings when exposed to a Woods lamp.

The papular form was the second most common form of EGCG in the study cohort, being diagnosed in 15 patients. This form presented as multiple erythematous to flesh-coloured, non-scaly, firm, indurated papules of diameter 2–12 mm distributed in groups, symmetrically or sporadically (Fig. 1g). These lesions were found in both sun-exposed and non-exposed areas. In the study cohort, some popular lesions were localized and some generalized.
Six patients presented with large, well-demarcated, diffuse, non-scaly, oedematous erythema plaques on the trunk; we classified these as the giant form (Fig. 1h). Some of the plaques were distributed symmetrically. Two of these patients reported pruritus, 3 had positive antinuclear antibodies, and 1 was diagnosed with Sjögren syndrome and interstitial pneumonia.
The condition was classified as mixed form in 15 patients, these patients having more than 2 of the types described above. All of these patients had a mixture of annular and papular lesions and 3 of them presented with generalized disease.
Histopathological findings
Despite the varying appearance of EGCG lesions clinically, the histopathological findings were consistent, including histiocytes and foreign-body type multinucleated cells engulfing elastotic fibres. Verhoeff-Van Gieson staining showed loss of elastic fibres in the dermis and phagocytosis of elastic fibres by histiocytic giant cells in all cases (Fig. 2a). A background of solar elastosis was sometimes found in the lesions in sun-exposed areas. The disease mainly involved the dermis and spared epidermis. We further identified 5 histopathological patterns in our cohort: giant cell (44, 41.9%) (Fig. 2b), necrobiotic (13, 12.4%) (Fig. 2c), histiocytic (32, 30.5%) (Fig. 2d), saicoidal (3, 2.8%) (Fig. 2e) and mixed patterns (13, 12.4%) (Fig 2f), the giant cell pattern being the most frequent. A combination of giant-cell and histiocytic patterns was the most common of the mixed forms. Alcian blue staining was performed to aid the differential diagnosis in 72 cases; no mucin deposition was found. No significant associations were found between different histopathological and clinical types (p = 0.917). We also found no significant correlation between pathological types and disease courses.
Comorbidities
Several comorbidities were identified at the time of diagnosed. Ten patients had DM, 5 a history of positive antinuclear antibodies, 4 impaired glucose tolerance, 4 hyperlipidaemia, 3 hypertension, 3 lung carcinoma, 1 chronic nephrotic syndrome, and 1 Sjögren syndrome and interstitial pneumonia.
Treatment and outcome
The patients had received various treatments, including oral glucocorticoids (0.5 mg/kg/day followed by tapering to none over 5 weeks to 5 months, n = 21), antihistamine (n = 7), hydroxychloroquine (n = 26), Tripterygium wilfordii (n = 13), glycyrrhizin (n = 1), topical corticosteroids (n = 24), and topical calcineurin inhibitors (n = 13). Sixty-seven patients were followed up by telephone, the remaining 38 patients being lost to follow-up. The lesions completely resolved in 19 patients, decreased in size or number in 33 patients, remained stable in 14, and worsened in one. Thirteen patients had recurrent lesions after a period of complete clearance. No severe drug-related adverse effects were observed. Among the patients whose lesions resolved completely, 12, 5 and 2 had been treated with systemic glucocorticoids, hydroxychloroquine and T. wilfordii, respectively. Topical corticosteroids (n = 17) or calcineurin inhibitors (n = 11) and oral hydroxychloroquine (n = 14) had achieved some control of the disease progression and reduction in number and severity of lesions. The lesion of 1 patient, who only received a topical corticosteroid, got worse. Recurrence of varying severity occurred within 1–3 years regardless of the medication prescribed. No correlations were found between EGCG subtypes and effectiveness of therapy.
DISCUSSION
EGCG, a granulomatous cutaneous disorder, is characterized by destruction of elastic fibres via phagocytosis by histiocytes and multinucleated giant cells in the dermis. The causes and pathogenesis of EGCG are controversial. It has been suggested that environmental factors (e.g. ultraviolet radiation, heat), host factors (systemic disorders, malignancies) or other unknown factors trigger the process, possibly by modifying the antigenicity of elastic fibres and precipitating cellular immunological reactions (10). Thereafter, dendritic cells or macrophages phagocytize elastin fragments to form granulomas or multinucleated giant cells. This theory is supported by the finding on immunohistochemical studies that CD4+ cells predominated over CD8+ cells in the inflammatory infiltrates (11). EGCG lesions reportedly do not occur in areas that are devoid of elastic fibres, such as burn scars or striae distensae (12, 13), supporting the hypothesis that elastic fibres are involved in pathogenesis. Upregulation of human matrix metalloproteinase-12, which is produced by macrophages and participates in degradation of elastic fibres and other structural components of the matrix, has recently been demonstrated in biopsy specimens of EGCG (13). It has therefore been postulated that matrix metalloproteinase-12 is involved in the dominant elastolysis process of EGCG.
Elastolytic giant cell granuloma has a large spectrum of clinical presentations
EGCG has been reported to occur predominantly in fair-skinned, elderly individuals, the female–male ratio ranging from 1.2:1 to 2:1; these data are consistent with the present findings (7). The disease characteristically runs a chronic course, ranging from months to years. Erythematous, annular plaques in the photosensitive areas are typical of EGCG (6). However, the current findings provide solid evidence that EGCG has varying clinical manifestations, lesions varying in size, number and shape and occurring in both sun-exposed and unexposed areas. The strong association between lesions and sun exposure supports the theory that phagocytosis of sun-damaged degenerated elastic fibres is involved in the pathogenesis of EGCG.
The shape of the skin lesions can change over time in patients with EGCG. Some patients initially present with papules that then coalesce gradually to form annular, polycyclic, arc-shaped, linear or imbricated plaques, with or without hypopigmented, atrophy centres.
In addition, one of the current patients had typical EGCG lesions that were confined to areas of vitiligo on the face; this has been rarely reported (5, 14). It has been postulated that oxidative stress and accumulation of free radicals trigger both of these conditions. In addition, cellular immunity is involved in the pathogenesis of both EGCG and vitiligo. The mechanism responsible for the association between these 2 conditions needs further investigation.
The current findings led us to propose a giant form of EGCG; 6 patients had this apparently rare subtype. Their lesions presented as giant, symmetrical or asymmetrical, erythematous plaques (diameter >10 cm) and most commonly involved covered areas. Antinuclear antibody positivity and/or a prior diagnosis of autoimmune diseases were identified in 67% of patients with the giant form of EGCG, suggesting that autoimmunity is involved in its pathogenesis. The specific mechanism requires further study. The giant form of EGCG can resemble GA, subacute cutaneous lupus erythematous, necrobiosis lipoidica, and erythema annulare centrifugum clinically; however, the histological findings differ. Misdiagnosis and missed diagnosis are very common because of the diversity and complexity of clinical manifestations. Only 17% of our study cohort had been clinically diagnosed as having EGCG. In view of the diverse clinical manifestations of the disease, which are not confined to annular lesions or actinic sites, we propose the term EGCG for this condition.
Elastolysis and elastophagocytosis are the main histological characteristics of elastolytic giant cell granuloma
Histologically, EGCG is characterized by granulomatous infiltration of the mid-dermis by multinucleated giant cells, histiocytes and lymphocytes (15). Some elastotic fibres were found in giant cells, proceeding to the phagocytosis and eliminating these damaged fibres. Damaged elastic fibres and elastosis are also characteristically present. Verhoeff-Van Gieson staining is critical to diagnosis of EGCG: it shows a marked loss of elastic fibres in major parts of the dermis and phagocytosis of elastic fibres by multinucleated giant cells (16). Our findings in this study led us to classify the pathological manifestations of EGCG into giant cell, necrobiotic, histiocytic, sarcoidal and mixed patterns. No statistical correlations were found between pathological type and clinical manifestations. With progression of this disease, histiocytes and multinuclear giant cells may gradually become more numerous, forming granulomatous infiltration and causing elastic fibre degeneration. We speculate that different pathological types may be associated with different course of the disease. However, we identified no such correlation in the current study, possibly because of the small number of some pathological types. Our theory needs to be further investigated in large-scale studies.
GA is the main clinical and histopathological differential diagnosis. This chronic, benign, inflammatory skin disease presents with arciform to annular plaques. Pathologically, GA is characterized by granulomas consisting of histiocytes and having necrobiotic foci distributed in interstitial or palisading patterns in the dermis. Multinuclear giant cells are sparse. Alcian blue staining shows mucin deposition among degenerated collagen fibres. Elastophagocytosis with diffuse loss of elastic tissue is not usually seen with GA.
Various comorbidities may occur in association with elastolytic giant cell granuloma
Associations between EGCG and DM, temporal arteritis, cutaneous amyloidosis, X-linked dominant protoporphyria and liver cirrhosis have been reported (6, 16–18). Definitive or latent DM are the most frequently related systemic disorders according to published reports (6). One previous study found that approximately 37% of Japanese patients with AEGCG have definite or latent DM (6). However, the association of AEGCG and DM remains controversial. It has been postulated that, in patients with diabetes, structural damage to elastic fibres caused by high serum concentrations of glucose and hyperglycaemic changes may precipitate inflammatory responses of EGCG. In the current study, DM and impaired glucose tolerance were the most commonly identified comorbidities, which is consistent with published reports. However, because this was a retrospective study, we had no data on latent DM. Associated malignancies had also been reported, including acute myelogenous leukaemia, adult T-cell leukaemia, prostate carcinoma, gastric carcinoma, oesophageal cancer and primary cutaneous T-cell lymphoma (8, 9, 19–21). EGCG lesions have reportedly regressed during treatment of associated malignancies (19). Given that the activity of the EGCG may parallel that of the malignancy, Gyldenløve et al. (8) believed that the co-existence of malignancies and EGCG may not be coincidental. It has been proposed that EGCG lesions represent a systemic immunological host defence against tumour antigens and that this condition might be a paraneoplastic phenomenon (16). The current study cohort included 3 patients who had been diagnosed with lung carcinoma before being diagnosed with EGCG diagnosis. To the best of our knowledge, this association has not previously been reported. Whether this was coincidental or related needs further investigation. Physicians should be alert that EGCG denotes the presence of an underlying malignancy and perform the necessary investigations.
Oral corticosteroid may be effective against elastolytic giant cell granuloma
EGCG characteristically has a chronic course and tends to resolve spontaneously without scaring over months to years. No standard treatment has yet been established. Patients should be instructed to rigorously protect their skin from sun. Treatment is based on limited case reports and series, reported treatments including topical, intralesional and systemic glucocorticoids, topical calcineurin inhibitors, methotrexate, hydroxychloroquine, minocycline, acitretin, dapsone, cyclosporine, ciclosporin, clofazimine, cryotherapy, psoralen ultraviolet A and narrowband ultraviolet B therapy (2–4, 6, 9, 11, 12, 22–24). The effectiveness of these treatment is variable. Some patients’ lesions achieved stable or subsided with sun-protection and topical glucocorticoids or calcineurin inhibitors, whether such resolution is spontaneous or drug-related remains uncertain. Oral corticosteroid therapy was the most effective treatment in the current study, 12 of 16 patients achieving complete resolution of their lesions. These findings are consistent with those of a previous study, which reported that low-dose glucocorticoids are effective against EGCG (25). The recurrence rate was approximately 19.4% in the current study. There is an urgent need to establish an effective treatment for this disease.
The limitations of this study include that this was a retrospective investigation in a single medical centre. The information on latent disease and long-term therapeutic outcomes was incomplete; further analysis of long-term data is required.
In conclusion, we present here the findings of a large retrospective study of EGCG, and suggest that variations in morphologies justify substituting the term EGCG for annular EGCG. It is essential to recognize the rare disease because of its possible association with systemic disorders. Such recognition would prompt adequate systemic evaluation and timely referral and treatment, minimizing the secondary effects of any comorbidities and improving the prognosis.
ACKNOWLEDGEMENTS
All authors participated in the design of the study and clinico-histological assessment. All the authors take responsibility for acquisition, analysis and interpretation of the data. DM had full access to all data in the study and takes responsibility for the integrity of data and accuracy of data analysis. YQ wrote the initial draft. JL and DM revised the manuscript. YT and TC reviewed the literature. WL and JL contributed to interpretation of the results and statistical analysis. The study was supervised by DM.
The authors have no conflicts of interest to declare.
REFERENCES
- Ma DL, Vano-Galvan S. Actinic Granuloma. N Engl J Med 2017; 376: 475.
- Rongioletti F, Baldari M, Burlando M, Parodi A. Papular elastolytic giant cell granuloma: report of a case associated with monoclonal gammopathy and responsive to topical tacrolimus. Clin Exp Dermatol 2010; 35: 145–148.
- Qian YT, Liu JW, Liu W, Ma DL. Image gallery: a reticular variant of actinic granuloma. Br J Dermatol 2019; 181: e90.
- Caldas R, Guimarães MJ, Rodrigues AP, Araújo C. Generalised papular variant of elastolytic giant cell granuloma. BMJ Case Rep 2019; 12: e231580.
- Watabe D, Akasaka T. Annular elastolytic giant cell granuloma developing on lesions of vitiligo. Int J Dermatol 2013; 52: 1458–1460.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol 2011; 36: 917–919.
- Parikh SA, Que SKT, Holmes WD, Ferenczi K, Grant-Kels JM, Rothe MJ. Infiltrated papules on the trunk and headaches: a case of actinic granuloma and a review of the literature. Int J Womens Dermatol 2015; 1: 131–135.
- Gyldenløve M, Faurschou A, Nielsen SL, Thyssen JP. Annular elastolytic giant cell granuloma in a patient with squamous cell carcinoma of the tonsil. JAAD Case Rep 2014; 1: 34–35.
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol 2017; 56: 738–745.
- Szabo SR, Fernelius C, Arora N. Annular elastolytic giant cell granuloma: mysterious enlarging scarring lesions. Cutis 2019; 103: E5–E7.
- Stefanaki C, Panagiotopoulos A, Kostakis P, Stefanaki K, Petridis A. Actinic granuloma successfully treated with acitretin. Int J Dermatol 2005; 44: 163–166.
- Ozkaya-Bayazit E, Büyükbabani N, Baykal C, Oztürk A, Okçu M, Soyer HP. Annular elastolytic giant cell granuloma: sparing of a burn scar and successful treatment with chloroquine. Br J Dermatol 1999; 140: 525–530.
- Asahina A, Shirai A, Horita A, Saito I. Annular elastolytic giant cell granuloma associated with prostate carcinoma: demonstration of human metalloelastase (MMP-12) expression. Clin Exp Dermatol 2012; 37: 70–72.
- de Paz NM, Rodríguez-Martín M, Bustínduy MG, Martín-Herrera A, Noda-Cabrera A. Strict anatomical colocalization of vitiligo and elastolytic granulomas. Case Rep Dermatol 2010; 2: 13–17.
- Gutierrez-Gonzalez E, Gomez-Bernal S, Alvarez-Perez A, Sanchez-Aguilar D, Toribio J. Elastolytic giant cell granuloma: clinic-pathologic review of twenty cases. Dermatol Online J 2013; 19: 20019.
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin 2015; 33: 331–341.
- García-Martínez FJ, Gutiérrez-González E, Alonso-González J, Vega A, Santamariña M, Rodríguez-Granados MT, et al. Annular elastolytic giant cell granuloma associated to late-onset X-linked dominant protoporphyria. Dermatology 2013; 227: 238–242.
- Shoimer I, Wismer J. Annular elastolytic giant cell granuloma associated with temporal arteritis leading to blindness. J Cutan Med Surg 2011; 15: 293–297.
- Garg A, Kundu RV, Plotkin O, Aronson IK. Annular elastolytic giant cell granuloma heralding onset and recurrence of acute myelogenous leukemia. Arch Dermatol 2006; 142: 532–533.
- Kuramoto Y, Watanabe M, Tagami H. Adult T cell leukemia accompanied by annular elastolytic giant cell granuloma. Acta Derm Venereol 1990; 70: 164–167.
- Michaelis TC, Woodcock JL, Basic KK. Actinic granuloma presenting as tender, linear plaques on the lateral fingers in a patient with newly diagnosed esophageal cancer. JAAD Case Rep 2017; 3: 55–57.
- Nanbu A, Sugiura K, Kono M, Muro Y, Akiyama M. Annular elastolytic giant cell granuloma successfully treated with minocycline hydrochloride. Acta Derm Venereol 2015; 95: 756–757.
- Liu W, Ma DL. Actinic granuloma. CMAJ 2019; 191: E895.
- Burlando M, Herzum A, Cozzani E, Paudice M, Parodi A. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? Case report and review of the literature. Dermatol Ther 2021; 34: e14705.
- Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol 2013; 40: 220–222.